The cerebellum constitutes 10 to 15 percent of the entire brain weight, about 140 grams in humans. Rollando (1809; see Dow and Moruzzi 1958) was the first who, by observing motor disturbances in an animal with a lesioned cerebellum, related the cerebellum to movement. Careful analyses of the motor disturbances so induced led Flourens (1824; see Dow and Moruzzi 1958) to conclude that the cerebellum is neither an initiator nor an actuator, but instead serves as a coordinator of movements. An animal with a damaged cerebellum still initiates and executes movement, but only in a clumsy manner. Flourens (1842) and Luciani (1891; see Dow and Moruzzi 1958) observed that motor disturbances caused in an animal by a partial lesion of the cerebellum were gradually compensated for due to the functional plasticity of cerebellar tissues. According to the current knowledge cited below, this plasticity is an expression of a learning capability of the cerebellum, which normally plays a role in MOTOR LEARNING, as in the cases of practicing sports and acquiring skilled movements. Early in the twentieth century, neurologists defined the unique symptoms, such as dysmetria and motor incoordination, of cerebellar diseases. Based on these classic observations, it has been thought that the major function of the cerebellum is to enable us to learn to perform movements accurately and smoothly. The extensive studies that have been performed over the past four decades have facilitated the formulation of comprehensive views on the structure of the cerebellum, what processes occur there, and what roles it plays not only in bodily but also in cognitive functions, even though some of the views are still hypothetical.
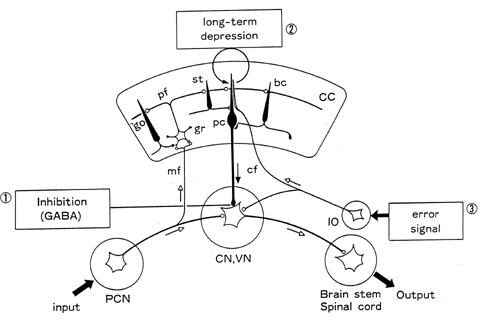
The cerebellar cortex contains an elaborate neuronal circuit composed of five types of cells: Purkinje, basket, strellate, Golgi, and granule cells (fig. 1; Eccles, Ito, and Szentagothai 1967). With respect to their synaptic action, these cells are inhibitory except for the granule cells, which are excitatory. Afferent signals from various precerebellar nuclei reach the cerebellar cortex and are relayed to the granule cells via mossy fibers, and to the dendrites of the Purkinje cells and other inhibitory cells via the axons of the granule cells, that is, parallel fibers. Afferent signals from the inferior olive in the medulla oblongata pass directly to the Purkinje cells via climbing fibers. L-glutamate is the neurotransmitter for the vast majority of mossy fibers and all granule cells, while GABA is the neurotransmitter for all inhibitory neurons.

Figure 1 Neuronal circuitry of the cerebellum. CC, cerebellar cortex; pc, Purkinje cell; bc, basket cell; st, stellate cell; pf, parallel fiber; gr, granule cell; go, Golgi cell; mf, mossy fiber; cf, climbing fiber; IO, inferior olive; PCN, precerebellar nuclei; CN, cerebellar nuclei; VN, vestibular nuclei.
The cerebellar cortex contains numerous small longitudinal microzones (Oscarsson 1976). Each microzone is paired with a small distinct group of neurons in a cerebellar or vestibular nucleus (CN, VN in fig. 1) to form a corticonuclear microcomplex that hereafter is referred to as a cerebellar chip or a chip (Ito 1984). In a cerebellar chip, input signals from various precerebellar nuclei activate the nuclear neurons that produce the output signals of the chip. This major signal path across a chip is attached with a sidepath through a microzone that receives input signals via mossy fibers and relays Purkinje cell signals to the nuclear neurons. Climbing fibers convey signals representing errors in the performance of the chip, as detected through various sensory pathways, which induce LTD in Purkinje cells. The LTD induction changes the signal flow through the microzone sidepath, thereby altering the signal flow across the chip. A cerebellar chip thus behaves as an adaptive unit in which input-output relationships are adaptively altered by error signals conveyed by climbing fibers.
The cerebellum is divided into the flocculonodular lobe and the corpus cerebelli, the latter being further divided into vermis, paravermis (intermediate part), and hemisphere. Cerebellar chips in the flocculonodular lobe, vermis, and paravermis are connected to the brain stem and spinal cord, and confer adaptiveness on reflexes (not only motor but also autonomic; for the vestibuloocular reflex, see Robinson 1976; Ito 1984; for eye-blink conditioned reflex, see Thompson 1987) and compound movements (for locomotion, see Yanagihara and Kondo 1996), which by themselves are stereotyped and nonadaptive. The role of the evolutionary old part of the cerebellum is therefore to ensure the adaptiveness of the spinal cord and brain stem control functions in order to enable animals to survive in ever-changing environments.
With respect to cerebral control functions, cerebellar chips appear to play a different role, that is, the formation of an internal model of a controller or a control object. If, while a chip and the system to be modeled are supplied with common input signals, differences in their output signals are returned to the chip as error signals, the chip will gradually assume dynamic characteristics equivalent to those of the system to be modeled.
Cerebellar chips located in the paravermis are connected to the cerebral motor cortex in such a way that these chips constitute a model that mimics the dynamics of the skeletomuscular system (Ito 1984). The motor cortex thus becomes capable of performing a learned movement with precision by referring to the model in the cerebellum and not to the skeletomuscular system. Dysmetria, the failure to perform a precise reaching movement without visual feedback, could be due to the loss of such internal models. Another possibility is that a chip located in the cerebellar hemisphere forms a model that acts as a controller in place of the motor cortex (Kawato, Furukawa, and Suzuki 1987; Shidara et al. 1995). Learned movements could then be controlled unconsciously, yet accurately, by the cerebellum. These two model systems, one eliminating the need for sensory feedback and the other awareness from learned voluntary movement control, appear to represent different phases of motor learning conducted in different cerebellar areas.
Based on the parallel development of the cerebral association cortex and cerebellar hemispheres in primates, Leiner, Leiner, and Dow (1986) suggested that the lateralmost part of the cerebellar hemisphere is involved in cognitive rather than motor functions. Thought may occur as a result of the prefrontal association cortex acting as a controller upon images, ideas, or concepts encoded in the parietolateral association cortex as a control object. During thought repetition, a cerebellar chip may form a model of the parietolateral cortex or the prefrontal cortex. A repeatedly learned thought may thus be performed quickly yet accurately even without reference to the consequences of the thought or without conscious attention. Evidence suggesting such roles of the cerebellum as this is accumulating from studies on the human cerebellum using noninvasive techniques (see Schmahmann 1997).
Albus, J. S. (1971). A theory of cerebellar function. Mathematical Bioscience 10:25-61.
Dow, R. E., and G. Moruzzi. (1958). The Physiology and Pathology of the Cerebellum. Minneapolis: University of Minnesota Press.
Eccles J. C., M. Ito, and J. Szentagothai. (1967). The Cerebellum as a Neuronal Machine. New York: Springer-Verlag.
Ito, M. (1984). The Cerebellum and Neural Control. New York: Raven Press.
Ito, M. (1989). Long-term depression. Annual Review of Neuroscience 12:85-102.
Ito, M. (1993). Movement and thought: identical control mechanisms by the cerebellum. Trends in Neuroscience 16:448-450.
Ito, M., M. Sakurai, and P. Tongroach. (1982). Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. Journal of Physiology, London 324:113-134.
Kawato, M., K. Furukawa, and R. Suzuki. (1987). A hierarchical neuronal network model for control and learning of voluntary movement. Biological Cybernetics 57:169-185.
Leiner, H. C., A. L. Leiner, and R. S. Dow. (1986). Does the cerebellum contribute to mental skill? Behavioral Neuroscience 100:443-453.
Marr, D. (1969). A theory of cerebellar cortex. Journal of Physiology, London 202:437-470.
Nakazawa, K., S. Mikawa, T. Hashikawa, and M. Ito. (1995). Transient and persistent phosphorylations of AMPA-type glutamate receptor subunits in cerebellar Purkinje cells. Neuron 1:697-709.
Oscarsson, O. (1976). Spatial distribution of climbing and mossy fibre inputs into the cerebellar cortex. In O. Creutzfeldt, Ed., Afferent and Intrinsic Organization of Laminated Structures in the Brain. Berlin: Springer-Verlag, pp. 34-42.
Robinson, D. A. (1976). Adaptive gain control of vestibulo-ocular reflex by the cerebellum. Journal of Neurophysiology 39:954-969.
Rosenblatt, F. (1962). Principles of Neurodynamics: Perceptron and the Theory of Brain Mechanisms. Washington, DC: Spartan Books.
Schmahmann, J. D. (1997). The Cerebellum and Cognition. San Diego: Academic Press.
Shidara, M., M. Kawano, H. Gomi, and M. Kawato. (1995). Inverse-dynamics encoding of eye movements by Purkinje cells in the cerebellum. Nature 365:50-52.
Thompson, R. F. (1987). The neurobiology of learning and memory. Science 233:941-947.
Yanagihara, D., and I. Kondo. (1996). Nitric oxide plays a key role in adaptive control of locomotion in cats. Proceedings of the National Academy of Sciences, USA 93:13292-13297.
Palay, S. L., and V. Chan-Palay. (1974). The Cerebellar Cortex. New York: Springer-Verlag.