Emission tomography is a visualization technique in nuclear medicine that yields an image of the distribution of a previously administered radionuclide in any desired transverse section of the body. Positron emission tomography (PET) utilizes the unique properties of the annihilation radiation generated when positrons are absorbed in matter. It is characterized by the fact that an image reconstructed from the radioactive counting data is an accurate and quantitative representation of the spatial distribution of a radionuclide in the chosen section. This approach is analogous to quantitative antoradiography performed in laboratory animals but has the added advantage of allowing in vivo studies and, hence, studies to be performed safely in human subjects.
PET, now along with MAGNETIC RESONANCE IMAGING (MRI), is at the forefront of cognitive neuroscience research in normal humans. The signal used by PET and MRI in this research is based on the fact that changes in the cellular activity of the brain of normal, awake humans and unanesthetized laboratory animals are invariably accompanied by changes in local blood flow (for reviews, see Raichle 1987, 1998). While PET measures blood flow directly, functional MRI or fMRI as it is now called, relies on the local changes in magnetic field properties occurring in the brain that result from changes in the blood flow that exceed changes in oxygen consumption (Raichle 1998). This is known as the blood oxygen level-dependent or BOLD signal.
This robust, empirical relationship between blood flow and brain function has fascinated scientists for well over a hundred years. One has only to consult William JAMES's monumental two-volume text Principles of Psychology (James 1890) on page 97 of the first volume to find reference to changes in brain blood flow during mental activities. He references primarily the work of the Italian physiologist Angelo Mosso (1881) who recorded the pulsation of the human cortex in patients with skull defects following neurosurgical procedures. Mosso showed that these pulsations increased regionally during mental activity and concluded, correctly we now know, that brain circulation changes selectively with neuronal activity.
At the close of World War II, Seymour Kety and his colleagues opened the modern era of studies of brain circulation and metabolism, introducing the first quantitative methods for measuring whole-brain blood flow and metabolism in humans. The introduction by Kety's group of an in vivo tissue autoradiographic measurement of regional blood flow applicable only in laboratory animals (Kety 1960; Landau et al. 1955) provided the first glimpse of quantitative changes in blood flow in the brain related directly to brain function. This work clearly foretold what was to come in the modern era of functional brain imaging with PET and MRI.
Soon after Kety and his colleagues introduced their quantitative methods for measuring whole-brain blood flow and metabolism in humans, David Ingvar, Neils Lassen, and their Scandinavian colleagues introduced methods applicable to humans that permitted regional blood flow measurements to be made using scintillation detectors arrayed like a helmet over the head (Lassen et al. 1963). They demonstrated directly in normal human subjects that blood flow changed regionally during changes in brain functional activity.
In 1973 Godfrey Hounsfield (Hounsfield 1973) introduced x-ray computed tomography (CT), a technique based upon principles presented in 1963 by Alan Cormack (Cormack 1963, 1973). Overnight the way in which we looked at the human brain changed. Immediately, researchers envisioned another type of tomography, positron emission tomography, or PET (Hoffman et al. 1976; Ter-Pogossian et al. 1975).
With the introduction of PET (Hoffman et al. 1976; Ter-Pogossian et al. 1975) a new era of functional brain mapping began. The autoradiographic techniques for the measurement of blood flow (Kety 1960; Landau et al. 1955) and glucose metabolism (Sokoloff et al. 1977) in laboratory animals could now be performed safely in humans (Raichle et al. 1983; Reivich et al. 1979).
Soon it was realized that highly accurate measurements of brain functional anatomy in humans could be performed with PET (Posner and Raichle 1994). While such functional brain imaging could be accomplished with either measurements of blood flow or metabolism (Raichle 1987), blood flow became the favored technique with PET because it could be measured quickly (in less than one minute) using an easily produced radiopharmaceutical (H 215O) with a short half-life (123 sec) which allowed many repeat measurements in the same subject (Raichle 1998).

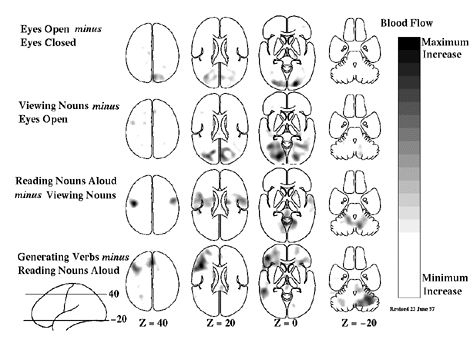
Figure 1 Four different hierarchically organized conditions are represented in these mean blood flow difference images obtained with PET. All of the changes shown in these images represent increases over the control state for each task. A group of normal subjects performed these tasks involving common English nouns (Petersen et al. 1988; Petersen et al. 1989) to demonstrate the spatially distributed nature of the processing by task elements going on in the normal human brain during a simple language task. Task complexity was increased from simply opening the eyes (row 1) through passive viewing of nouns on the television monitor (row 2); reading aloud the nouns as they appear on the screen (row 3); and saying aloud an appropriate verb for each noun as it appeared on the screen (row 4). These horizontal images are oriented with the front of the brain on top and the left side to the reader's left. The marking "Z = 40" indicates milimeters above and below a horizontal plane through the brain marked "Z = 40".
The study of human cognition with PET was aided greatly by the involvement of cognitive psychologists in the 1980s whose experimental designs for dissecting human behaviors using information processing theory fit extremely well with the emerging functional brain imaging strategies (Posner and Raichle 1994). As a result of collaboration among neuroscientists, imaging scientists, and cognitive psychologists, a distinct behavioral strategy for the functional mapping of neuronal activity emerged. This strategy was based on a concept introduced by the Dutch physiologist Franciscus C. Donders in 1868 (reprinted in Donders 1969). Donders proposed a general method of measuring thought processes based on a simple logic. He subtracted the time needed to respond to a light (say, by pressing a key) from the time needed to respond to a particular color of light. He found that discriminating color required about 50 msec. In this way, Donders isolated and measured a mental process for the first time by subtracting a control state (i.e., responding to a light) from a task state (i.e., discriminating the color of the light). This strategy (figure 1) was first introduced to functional brain imaging with PET in the study of single-word processing (Petersen et al. 1988, 1989, 1990) but quickly became the dominant approach to the study of all aspects of human cognition with functional brain imaging.
One criticism of this subtractive approach has been that the time necessary to press a key after a decision to do so has been made, for instance, is affected by the nature of the decision process itself. By implication, the nature of the processes underlying key press, in this example, may have been altered. Although this issue (known in cognitive science jargon as the assumption of pure insertion) has been the subject of continuing discussion in cognitive psychology, it finds its resolution in functional brain imaging, where changes in any process are directly signaled by changes in observable brain states. Events occurring in the brain are not hidden from the investigator as in the purely cognitive experiments. Careful analysis of the changes in the functional images reveals whether processes (e.g., specific cognitive decisions) can be added or removed without affecting ongoing processes (e.g., motor processes). Processing areas of the brain whose activity is differentially altered at various stages of a hierarchically organized cognitive paradigm can be readily seen with imaging (figure 2). Clearly, extant data now provide many examples of areas of the brain active at one stage in a hierarchically designed paradigm which become inactive as task complexity is increased (for a recent review, see Raichle 1998). While changes of this sort are hidden from the view of the cognitive scientist they become obvious when brain imaging is employed.
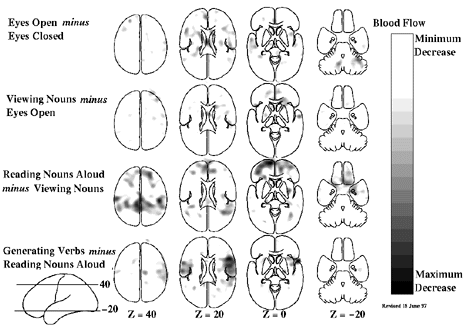
Figure 2 Hierarchically organized subtraction involving the same task conditions as shown in Figure 1 with the difference being that these images represent areas of decreased activity in the condition as compared with the control condition. Combining the information available in Figures 1 and 2 provides a fairly complete picture of the interactions between tasks and brain systems in hierarchically organized cognitive tasks when studied with functional brain imaging.
A final caveat with regard to imaging certain cognitive paradigms is that the brain systems involved do not necessarily remain constant through many repetitions of the task (e.g., see Raichle et al. 1994; Raichle 1998). While simple habituation might be suspected when a task is tedious, this is not the issue referred to here. Rather, when a task is novel and, more importantly, conflicts with a more habitual response to the presented stimulus, major changes can occur in the systems allocated to the task. Such changes have both practical and theoretical implications when it comes to the design and interpretation of cognitive activation experiments.
Functional brain imaging provides a unique perspective on the relationship between brain function and behavior in humans that is unavailable in the purely cognitive experiments and, in many instances, unattainable in experiments restricted to laboratory animals. fMRI has greatly expanded the work initiated with PET owing to its better spatial and temporal resolution. Using fMRI it is now possible, for example, to image the brain changes associated with single cognitive events in individual subjects (Buckner et al. 1996).
One of the great challenges remaining in the use of functional imaging with either PET or MRI is to understand more fully the relationship between brain blood flow and brain function (Raichle 1998).
Buckner, R. L., P. A. Bandettini, K. M. O'Craven, R. L. Savoy, S. E. Petersen, M. E. Raichle, and B. R. Rosen. (1996). Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proceedings of the National Academy of Sciences 93:14878-14883.
Cormack, A. M. (1963). Representation of a function by its line integrals, with some radiological physics. Journal of Applied Physics 34:2722-2727.
Cormack, A. M. (1973). Reconstruction of densities from their projections, with applications in radiological applications. Phys. Med. Biol. 18:195-207.
Donders, F. C. (1869/1969). On the speed of mental processes. Acta Psychologia 30:412-431.
Hoffman, E. J., M. E. Phelps, N. A. Mullani, C. S. Higgins, and M. M. Ter-Pogossian. (1976). Design and performance characteristics of a whole-body positron tranxial tomograph. Journal of Nuclear Medicine 17:493-502.
Hounsfield, G. N. (1973). Computerized transverse axial scanning (tomography): Part I. Description of system. British Journal of Radiology 46:1016-1022.
James, W. (1890). Principles of Psychology. New York: Henry Holt, pp. 97-99.
Kety, S. (1960). Measurement of local blood flow by the exchange on an inert diffusible substance. Methods in Medical Research 8:228-236.
Landau, W. M., W. H. Freygang Jr., L. P. Roland, L. Sokoloff, and S. Kety. (1955). The local circulation of the living brain: Values in the unanesthetized and anesthetized cat. Transactions of the American Neurological Association 80:125-129.
Lassen, N. A., K. Hoedt-Rasmussen, S. C. Sorensen, E. Skinhoj, B. Cronquist, E. Bodforss, and D. H. Ingvar. (1963). Regional cerebral blood flow in man determined by Krypton-85. Neurology 13:719-727.
Mosso, A. (1881). Über den Kreislauf des Blutes im menschlichen Gehirn. Leipzig: Verlag von Veit.
Petersen, S. E., R. T. Fox, M. I. Posner, M. Mintum, and M. E. Raichle. (1988). Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 331:585-589.
Petersen, S. E., P. T. Fox, M. I. Posner, M. A. Mintun, and M. E. Raichle. (1989). Positron emission tomographic studies of the processing of single words. Journal of Cognitive Neuroscience 1:153-170.
Petersen, S. E., P. T. Fox, A. Z. Snyder, and M. E. Raichle. (1990). Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science 249:1041-1044.
Posner, M. I., and M. E. Raichle. (1994). Images of Mind. New York: W. H. Freeman.
Raichle, M. E. (1987). Circulatory and metabolic correlates of brain function in normal humans. In F. Plum, Ed., Handbook of Physiology: The Nervous System V. Higher Functions of the Brain. Bethesda, MD: American Physiological Society, pp. 643-674.
Raichle, M. E. (1998). Behind the scenes of function brain imaging: A historical and physiological perspective. Proceedings of the National Academy of Sciences 95:765-772.
Raichle, M. E., W. R. W. Martin, P. Herscovitch, M. A. Mintun, and J. Markham. (1983). Brain blood flow measured with intravenous H215O. 2. Implementation and validation. Journal of Nuclear Medicine 24:790-798.
Raichle, M. E., J. A. Fiez, T. O. Videen, A. K. MacLeod, J. V. Pardo, P. T. Fox, and S. E. Petersen. (1994). Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex 4:8-26.
Reivich, M., D. Kuhl, A. Wolf, J. Greenberg, M. Phelps, T. Ido, V. Casella, E. Hoffman, A. Alavi, and L. Sokoloff. (1979). The [18F] flourodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circulation Research 44:127-137.
Sokoloff, L., M. Reivich, C. Kennedy, M. H. Des Rosiers, C. S. Patlak, K. D. Pettigrew, O. Sakurada, and M. Shinohara. (1977). The [14C]deoxyglucose method for the measurement of local glucose utilization: Theory, procedure and normal values in the conscious and anesthetized albino rat. Journal of Neurochemistry 28:897-916.
Ter-Pogossian, M. M., M. E. Phelps, E. J. Hoffman, and N. A. Mullani. (1975). A positron-emission tomograph for nuclear imaging (PET). Radiology 114:89-98.