Binding by Neural Synchrony
Neuronal
systems have to solve immensely complex combinatorial problems and
require efficient binding mechanisms in order to generate representations
of perceptual objects and movements. In the context of cognitive
functions, combinatorial problems arise from the fact that perceptual
objects are defined by a unique constellation of features, the diversity
of possible constellations being virtually unlimited (cf. BINDING PROBLEM). Combinatorial problems of similar magnitude
have to be solved for the acquisition and execution of motor acts.
Although the elementary components of motor acts, the movements
of individual muscle fibers, are limited in number, the spatial
and temporal diversity of movements that can be composed by combining
the elementary components in ever-changing constellations is again
virtually infinite. In order to establish neuronal representations
of perceptual objects and movements, the manifold relations among
elementary sensory features and movement components have to be encoded
in neural responses. This requires binding mechanisms that can cope
efficiently with combinatorial complexity. Brains have acquired
an extraordinary competence to solve such combinatorial problems,
and it appears that this competence is a result of the evolution
of the CEREBRAL CORTEX.
In the primary visual cortex of mammals,
relations among the responses of retinal ganglion cells are evaluated
and represented by having the output of selected arrays of ganglion
cells converge in diverse combinations onto individual cortical
neurons. Distributed signals are bound together by selective convergence
of feed forward connections (Hubel and Wiesel 1962). This strategy
is iterated in prestriate cortical areas in order to generate neurons
that detect and represent more complex constellations of features
including whole perceptual objects.
However, this strategy of binding
features together by recombining input connections in ever-changing
variations and representing relations explicitly by responses of
specialized cells results in a combinatorial explosion of the number
of required binding units. It has been proposed, therefore, that
the cerebral cortex uses a second, complementary strategy, commonly called
assembly coding, that permits utilization of the same set of neurons
for the representation of different relations (Hebb 1949). Here,
a particular constellation of features is represented by the joint
and coordinated activity of a dynamically associated ensemble of
cells, each of which represents explicitly only one of the more
elementary features that characterize a particular perceptual object.
Different objects can then be represented by recombining neurons
tuned to more elementary features in various constellations (assemblies). For
assembly coding, two constraints need to be met. First, a selection
mechanism is required that permits dynamic, context dependent association
of neurons into distinct, functionally coherent assemblies. Second,
grouped responses must get labeled so that they can be distinguished
by subsequent processing stages as components of one coherent representation
and do not get confounded with other unrelated responses. Tagging
responses as related is equivalent with raising their salience jointly
and selectively, because this assures that they are processed and
evaluated together at the subsequent processing stage. This can
be achieved in three ways. First, nongrouped responses can be inhibited;
second, the amplitude of the selected responses can be enhanced;
and third, the selected cells can be made to discharge in precise temporal
synchrony. All three mechanisms enhance the relative impact of the
grouped responses at the next higher processing level. Selecting
responses by modulating discharge rates is common in labeled line
coding where a particular cell always signals the same content.
However, this strategy may not always be suited for the distinction
of assemblies because it introduces ambiguities, reduces processing
speed, and causes superposition problems (von der Malsburg 1981; Singer
et al. 1997). Ambiguities could arise because discharge rates of
feature-selective cells vary over a wide range as a function of
the match between stimulus and receptive field properties; these
modulations of response amplitude would not be distinguishable from
those signalling the relatedness of responses. Processing speed
would be reduced because rate coded assemblies need to be maintained
for some time in order to be distinguishable. Finally, superposition
problems arise, because rate coded assemblies cannot overlap in
time within the same processing stage. If they did, it would be
impossible to distinguish which of the enhanced responses belong
to which assembly. Simultaneous maintenance of different assemblies
over perceptual time scales is required, however, to represent composite
objects.
Both the ambiguities and the temporal
constraints can be overcome if the selection and labeling of responses
is achieved through synchronization of individual discharges (Gray
et al. 1989; Singer and Gray 1995). Synchronicity can be adjusted
independently of rates, and so the signature of relatedness, if expressed
through synchronization, is independent of rate fluctuations. Moreover,
synchronization enhances only the salience of those discharges that
are precisely synchronized and generate coincident synaptic potentials
in target cells at the subsequent processing stage. Hence the selected
event is the individual spike or a brief burst of spikes. Thus,
the rate at which different assemblies can follow one another within the
same neuronal network without getting confounded is much higher
than with rate coding. It is only limited by the duration of the
interval over which synaptic potentials summate effectively. If
this interval is in the range of 10 or 20 ms, several different
assemblies can alternate within preceptually relevant time windows.
If synchronization serves as a selection
and binding mechanism, neurons must be sensitive to coincident input.
Moreover, synchronization must occur rapidly and show a relation
to perceptual phenomena.
Although the issue of coincidence
detection is still controversial (König, Engel, and Singer
1996; Shadlen and Newsome 1994), evidence is increasing that neurons
can evaluate temporal relations with precision among incoming activity (see
e.g., Carr 1993). That cortical networks can handle temporally structured
activity with high precision and low dispersion follows from the
abundant evidence on the oscillatory patterning and precise synchronization
of neuronal responses in the
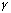 -frequency range
(Singer and Gray 1995; König, Engel, and Singer 1996).
Synchronization at such high frequencies is only possible if integration
time constants are short. Precise synchronization over large distances
is usually associated with an oscillatory patterning of responses
in the
-frequency range
(Singer and Gray 1995; König, Engel, and Singer 1996).
Synchronization at such high frequencies is only possible if integration
time constants are short. Precise synchronization over large distances
is usually associated with an oscillatory patterning of responses
in the 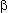 -
and
-
and 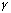 -frequency
range, suggesting a causal relation (König, Engel, and
Singer 1995). This oscillatory patterning is associated with strong
inhibitory interactions (Traub et al. 1996), raising the possibility
that the oscillations contribute to the shortening of integration
time constants.
-frequency
range, suggesting a causal relation (König, Engel, and
Singer 1995). This oscillatory patterning is associated with strong
inhibitory interactions (Traub et al. 1996), raising the possibility
that the oscillations contribute to the shortening of integration
time constants.
Simulations with spiking neurons
reveal that networks of appropriately coupled units can undergo
very rapid transitions from uncorrelated to synchronized states
(Deppisch et al. 1993; Gerstner 1996). Rapid transitions from independent
to synchronized firing are also observed in natural networks. In visual
centers, it is not uncommon that neurons engage in synchronous activity,
often with additional oscillatory patterning, at the very same time
they increase their discharge rate in response to the light stimulus
(Neuenschwander and Singer 1996; Gray et al. 1992). One mechanism
is coordinated spontaneous activity that acts like a dynamic filter
and causes a virtually instantaneous synchronization of the very first
discharges of responses Fries et al. 1997b). The spatio-temporal
patterns of these spontaneous fluctuations of excitability reflect
the architecture and the actual functional state of intracortical
association connections. Thus, grouping by synchronization can be
extremely fast and still occur as a function of both the prewired
associational dispositions and the current functional state of the
cortical network.
Evidence indicates that the probability
and strength of response synchronization reflects elementary Gestalt
criteria such as continuity, proximity, similarity in the orientation
domain, colinearity, and common fate (Gray et al. 1989; Engel, König,
and Singer 1991; Engel et al. 1991; Freiwald, Kreiter, and Singer 1995;
Kreiter and Singer 1996). Most importantly, the magnitude of synchronization
exceeds that expected from stimulus-induced rate covariations of
responses, indicating that it results from internal coordination
of spike timing. Moreover, synchronization probability does not
simply reflect anatomical connectivity but changes in a context-dependent
way (Gray et al. 1989; Engel, König, and Singer 1991; Freiwald, Kreiter,
and Singer 1995; Kreiter and Singer 1996), indicating that it is
the result of a dynamic and context-dependent selection and grouping
process. Most of the early experiments on response synchronization
have been performed in anesthetized animals, but more recent evidence
from cats and monkeys indicates that highly precise, internally
generated synchrony occurs also in the awake brain, exhibits similar
sensitivity to context (Kreiter and Singer 1996; Fries et al. 1997a;
Gray and Viana Di Prisco 1997), and is especially pronounced when
the EEG is desynchronized (Munk et al. 1996) and the animals are
attentive (Roelfsema et al. 1997). Direct relations between response
synchronization and perception have been found in cats who suffered
from strabismic amblyopia, a developmental impairment of vision associated
with suppression of the amblyopic eye, reduced visual acuity, and
disturbed perceptual grouping (crowding) in this eye. Quite unexpectedly,
the discharge rates of individual neurons in the primary visual
cortex fail to reflect these deficits (see Roelfsema et al. 1994
for references). The only significant correlate of amblyopia is
the drastically reduced ability of neurons driven by the amblyopic
eye to synchronize their responses (Roelfsema et al. 1994), and
this accounts well for the perceptual deficits: by reducing the salience
of responses, disturbed synchronization could be responsible for
the suppression of signals from the amblyopic eye, and by impairing
binding, it could reduce visual acuity and cause crowding.
Another close correlation between
response synchronization and perception has been found in experiments
on binocular rivalry (Fries et al. 1997a). A highly significant
correlation exists between changes in the strength of response synchronization
in primary visual cortex and the outcome of rivalry. Cells mediating
responses of the eye that won in interocular competition increased
the synchronicity of their responses upon presentation of the rival
stimulus to the other, losing eye, while the reverse was true for
cells driven by the eye that became suppressed.
These results support the hypothesis
that precise temporal relations between the discharges of spatially
distributed neurons matter in cortical processing and that synchronization
may be exploited to jointly raise the salience of the responses selected
for further processing, that is, for the dynamic binding of distributed
responses into coherent assemblies.
The example of rivalry also illustrates
how synchronization and rate modulation depend on each other. The
signals from the suppressed eye failed to induce tracking EYE MOVEMENTS,
indicating that the vigorous but poorly synchronized responses in
primary visual areas eventually failed to drive the neurons responsible
for the execution of eye movements. Thus, changes of synchronicity result
in changes of response amplitudes at subsequent processing stages.
This convertibility provides the option to use both coding strategies
in parallel in order to encode complementary information.
See also
-- Wolf Singer
References
Carr, C. E. (1993). Processing of temporal information
in the brain. Annu. Rev. Neurosci. 16:223-243.
Deppisch, J., H.-U. Bauer, T. B. Schillen, P.
König, K. Pawelzik, and T. Geisel. (1993). Alternating
oscillatory and stochastic states in a network of spiking neurons. Network 4:243-257.
Engel, A. K., P. König, and W. Singer. (1991).
Direct physiological evidence for scene segmentation by temporal
coding. Proc. Natl. Acad. Sci. USA 88:9136-9140.
Engel, A. K., A. K. Kreiter, P. König, and
W. Singer. (1991). Synchronization of oscillatory neuronal responses
between striate and extrastriate visual cortical areas of the cat. Proc.
Natl. Acad. Sci. USA 88:6048-6052.
Freiwald, W. A., A. K. Kreiter, and W. Singer.
(1995). Stimulus dependent intercolumnar synchronization of single
unit responses in cat area 17. Neuroreport 6:2348-2352.
Fries, P., P. R. Roelfsema, A. K. Engel, P.
König, and W. Singer. (1997a). Synchronization of oscillatory
responses in visual cortex correlates with perception in interocular
rivalry. Proc. Natl. Acad. Sci. USA 94:12699-12704.
Fries, P., P. R. Roelfsema, W. Singer, and A.
K. Engel. (1997b). Correlated variations of response latencies due
to synchronous subthreshold membrane potential fluctuations in cat
striate cortex. Soc. Neurosci. Abstr. 23: 1266.
Gerstner, W. (1996). Rapid phase locking in
systems of pulse-coupled oscillators with delays. Phys. Rev.
Lett. 76:1755-1758.
Gray, C. M., A. K. Engel, P. König, and
W. Singer. (1992). Synchronization of oscillatory neuronal responses
in cat striate cortex -- temporal properties. Visual
Neurosci. 8:337-347.
Gray, C. M., P. König, A. K. Engel, and
W. Singer. (1989). Oscillatory responses in cat visual cortex exhibit
inter-columnar synchronization which reflects global stimulus properties. Nature 338:334-337.
Gray, C. M., and G. Viana Di Prisco. (1997).
Stimulus-dependent neuronal oscillations and local synchronization
in striate cortex of the alert cat. J. Neurosci. 17:3239-3253.
Hebb, D. O. (1949). The Organization of
Behavior. Wiley.
Hubel, D. H., and T. N. Wiesel. (1962). Receptive
fields, binocular interaction and functional architecture in the
cat's visual cortex. J. Physiol. (Lond.) 160:106-154.
König, P., A. K. Engel, and W. Singer. (1995).
Relation between oscillatory activity and long-range synchronization
in cat visual cortex. Proc. Natl. Acad. Sci. USA 92:290-294.
König, P., A. K. Engel, and W. Singer. (1996).
Integrator or coincidence detector? The role of the cortical neuron
revisited. Trends Neurosci. 19:130-137.
Kreiter, A. K., and W. Singer. (1996). Stimulus-dependent
synchronization of neuronal responses in the visual cortex of awake
macaque monkey. J. Neurosci. 16:2381-2396.
Munk, M. H. J., P. R. Roelfsema, P. König,
A. K. Engel, and W. Singer. (1996). Role of reticular activation
in the modulation of intracortical synchronization. Science 272:271-274.
Neuenschwander, S., and W. Singer. (1996). Long-range
synchronization of oscillatory light responses in the cat retina
and lateral geniculate nucleus. Nature 379:728-733.
Roelfsema, P. R., A. K. Engel, P. König,
and W. Singer. (1997). Visuomotor integration is associated with
zero time-lag synchronization among cortical areas. Nature 385:157-161.
Roelfsema, P. R., P. König, A. K. Engel,
R. Sireteanu, and W. Singer. (1994). Reduced synchronization in
the visual cortex of cats with strabismic amblyopia. Eur.
J. Neurosci. 6:1645-1655.
Shadlen, M. N., and W. T. Newsome. (1994). Noise,
neural codes and cortical organization. Curr. Opin. Neurobiol. 4:569-579.
Singer, W., A. K. Engel, A. K. Kreiter, M. H.
J. Munk, S. Neuenschwander, and P. R. Roelfsema. (1997). Neuronal
assemblies: necessity, signature and detectability. Trends
Cognitive Sci. 1(7):252-261.
Singer, W., and C. M. Gray. (1995). Visual feature
integration and the temporal correlation hypothesis. Annu.
Rev. Neurosci. 18:555-586.
Traub, R. D., M. A. Whittington, I. M. Stanford,
and J. G. R. Jefferys. (1996). A mechanism for generation of long-range
synchronous fast oscillations in the cortex. Nature 383:621-624.
von der Malsburg, C. (1981). The correlation
theory of brain function. Internal Report 81-2 Max-Planck-Institute
for Biophysical Chemistry. Reprinted 1994, in E. Domany, J. L. van
Hemmen and K. Schulten, Eds., Models of Neural Networks II.
Springer-Verlag, pp. 95-119.
Further Readings
Abeles, M. (1991). Corticonics. Cambridge:
Cambridge University Press.
Abeles, M., Y. Prut, H. Bergman, and E. Vaadia.
(1994). Synchronization in neuronal transmission and its importance
for information processing. Prog. Brain Res. 102:395-404.
Barlow, H. B. (1972). Single units and sensation:
a neuron doctrine for perceptual psychology? Perception 1:371-394.
Bauer, H.-U., and K. Pawelzik. (1993). Alternating
oscillatory and stochastic dynamics in a model for a neuronal assembly. Physica
D. 69:380-393.
Braitenberg, V. (1978). Cell assemblies in the
cerebral cortex. In R. Heim and G. Palm, Eds., Architectonics
of the Cerebral Cortex. Lecture Notes in Biomathematics 21, Theoretical
Approaches in Complex Systems. Springer-Verlag, pp. 171-188.
Edelman, G. M. (1987). Neural Darwinism:
The Theory of Neuronal Group Selection. New York: Basic Books.
Engel, A. K., P. König, A. K. Kreiter, T.
B. Schillen, and W. Singer. (1992). Temporal coding in the visual
cortex: new vistas on integration in the nervous system. Trends
Neurosci.15:218-226.
Frien, A., R. Eckhorn, R. Bauer, T. Woelbern,
and H. Kehr. (1994). Stimulus-specific fast oscillations at zero
phase between visual areas V1 and V2 of awake monkey. Neuroreport 5:2273-2277.
Gerstein, G. L., and P. M. Gochin. (1992). Neuronal
population coding and the elephant. In A. Aertsen and V. Braitenberg,
Eds., Information Processing in the Cortex, Experiments and
Theory. Springer-Verlag, pp. 139-173.
Gerstner, W., R. Kempter, J. L. van Hemmen,
and H. Wagner. (1996). A neuronal learning rule for sub-millisecond
temporal coding. Nature 383:76-78.
Gerstner, W., and J. L. van Hemmen. (1993).
Coherence and incoherence in a globally coupled ensemble of pulse-emitting
units. Phys. Rev. Lett. 7:312-315.
Hopfield, J. J., and A. V. M. Hertz. (1995).
Rapid local synchronization of action potentials: toward computation
with coupled integrate-and-fire neurons. Proc. Natl. Acad.
Sci. USA 92:6655-6662.
Jagadeesh, B., H. S. Wheat, and D. Ferster.
(1993). Linearity of summation of synaptic potentials underlying
direction selectivity in simple cells of the cat visual cortex. Science 262:1901-1904.
Löwel, S., and W. Singer. (1992). Selection
of intrinsic horizontal connections in the visual cortex by correlated
neuronal activity. Science 255:209-212.
Morgan, M. J., and E. Castet. (1995). Stereoscopic
depth perception at high velocities. Nature 378:380-383.
Palm, G. (1990). Cell assemblies as a guideline
for brain research. Concepts Neurosci. 1:133-147.
Phillips, W. A., and W. Singer. (1997). In search
of common foundations for cortical computation. Behav. Brain
Sci. 20(4):657-722.
Schmidt, K., R. Goebel, S. Löwel, and
W. Singer. (1997). The perceptual grouping criterion of colinearity
is reflected by anisotropies of connections in the primary visual
cortex. Europ. J. Neurosci. 9:1083-1089.
Singer, W. (1993). Synchronization of cortical
activity and its putative role in information processing and learning. Annu.
Rev. Physiol. 55:349-374.
Singer, W. (1995). Development and plasticity
of cortical processing architectures. Science 270:758-764.
Singer, W. (1996). Neuronal synchronization:
a solution to the binding problem? In R. Llinas and P.S. Churchland,
Eds., The Mind-Brain Continuum. Sensory Processes. Cambridge:
MIT Press, pp. 100-130.
Singer, W., A. K. Engel, A. K. Kreiter, M. H.
J. Munk, S. Neuenschwander, and P. R. Roelfsema. (1997). Neuronal
assemblies: necessity, signature and detectability. Trends
in Cognitive Sciences 1(7):252-261.
Softky, W. R. (1995). Simple codes versus efficient
codes. Curr. Opin. Neurobiol. 5:239-247.
Tallon-Baudry, C., O. Bertrand, C. Delpuech,
and J. Pernier. (1996). Stimulus specificity of phase-locked and
non-phase-locked 40 Hz visual responses in human. J. Neurosci. 16:4240-4249 .
 Copyright © 1999 Massachusetts Institute of Technology
Copyright © 1999 Massachusetts Institute of Technology
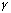 -frequency range
(Singer and Gray 1995; König, Engel, and Singer 1996).
Synchronization at such high frequencies is only possible if integration
time constants are short. Precise synchronization over large distances
is usually associated with an oscillatory patterning of responses
in the
-frequency range
(Singer and Gray 1995; König, Engel, and Singer 1996).
Synchronization at such high frequencies is only possible if integration
time constants are short. Precise synchronization over large distances
is usually associated with an oscillatory patterning of responses
in the 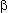 -
and
-
and 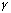 -frequency
range, suggesting a causal relation (König, Engel, and
Singer 1995). This oscillatory patterning is associated with strong
inhibitory interactions (Traub et al. 1996), raising the possibility
that the oscillations contribute to the shortening of integration
time constants.
-frequency
range, suggesting a causal relation (König, Engel, and
Singer 1995). This oscillatory patterning is associated with strong
inhibitory interactions (Traub et al. 1996), raising the possibility
that the oscillations contribute to the shortening of integration
time constants.