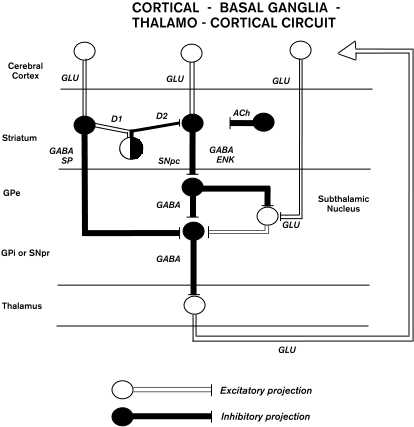
The CEREBRAL CORTEX is massively interconnected with a large group of subcortical structures known as the "basal ganglia." In general, the basal ganglia can be described as a set of input structures that receive direct input from the cerebral cortex, and output structures that project back to the cerebral cortex via the THALAMUS. Thus a major feature of basal ganglia anatomy is their participation in multiple loops with the cerebral cortex, termed cortico-basal ganglia-thalamo-cortical circuits (see Alexander, DeLong, and Strick 1986, figure 1).
Although the term basal ganglia was first used to indicate the putamen and globus pallidus (Ringer 1879), it now refers to the striatum, globus pallidus, subthalamic nucleus (STN), and substantia nigra. The striatum has three subdivisions, the caudate, putamen, and ventral striatum, that together form the main input structures of the basal ganglia. The globus pallidus consists of an external segment (GPe) and an internal segment (GPi). The GPe and STN are thought to represent "intermediate" basal ganglia structures, although the STN also receives some direct cortical inputs. The substantia nigra comprises two major cell groups, the pars compacta (SNpc) and pars reticulata (SNpr). The SNpr and GPi are the major output structures of the basal ganglia.
There has been considerable progress in defining the intrinsic organization of basal ganglia circuits (see Parent and Hazrati 1995). Briefly, inputs from the cerebral cortex to the striatum use glutamate (GLU) as an excitatory neurotransmitter to synapse on medium-sized (12-20 m) spiny stellate neurons, which are also the projection or output neurons of the striatum. Most of the cortical input terminates in striatal regions known as the "matrix," which contain high levels of acetylcholinesterase, the enzyme responsible for breaking down the neurotransmitter acetylcholine (Ragsdale and Graybiel 1981). Efferents from other cortical areas terminate in striatal regions termed "patches" or "striosomes," which have low levels of acetylcholinesterase. In addition to these differences in afferent input, the medium spiny stellate cells in the striosomes and matrix also have different efferent connections. Output cells in striosomes project to neurons in SNpc that produce the neurotransmitter dopamine. The axons of these SNpc cells project back to the striatum, where they release dopamine. The net effect of dopamine on striatal cells depends on the type of receptors present.
Output neurons in the matrix project to GPe, GPi, or SNpr. These striatal cells use the neurotransmitter gamma-aminobutyric acid (GABA) to inhibit their targets. The matrix cells projecting to GPi or SNpr express high levels of a neuropeptide called "substance P (SP)" and are excited by the action of dopamine on their D1 receptors. In contrast, matrix cells projecting to GPe express high levels of enkephalin (ENK) and are inhibited by the action of dopa mine on their D2 receptors (figure 1).
Efferents from GPe project largely to the STN, GPi and SNpr and use GABA to inhibit their targets. Neurons in the STN project to GPi or SNpr where they use GLU to excite neurons in both structures (figure 1). There is also evidence for GPe projections to the reticular nucleus of the thalamus, but the significance of this projection is unknown.
Neurons in the GPi and SNpr are the principal outputs of the basal ganglia. These neurons innervate a specific set of thalamic nuclei and use GABA as an inhibitory transmitter. Output neurons in the thalamus that receive basal ganglia input use GLU as a neurotransmitter to excite their targets. Although some of these thalamic neurons project back to the striatum, and thus form a closed feedback loop with the basal ganglia, the major output from the basal ganglia is to thalamic neurons that in turn project to the cerebral cortex. This pathway forms the efferent limb of the cortico-basal ganglia-thalamocortical circuit. Output neurons in SNpr and GPi also project to brain stem nuclei such as the superior colliculus and pedunculopontine nucleus. The projection to the colliculus appears to play a role in the generation of eye and head movements. The function of the pedunculopontine projection is more obscure. Pedunculopontine neurons appear to largely project back upon SNpc, GPi, and STN.
Figure 1

Recently, there have been some dramatic changes in concepts about the function of basal ganglia loops with the cerebral cortex. These loops were thought to collect inputs from widespread cortical areas in the frontal, parietal, and temporal lobes and to "funnel" this information back to the primary motor cortex or other cortical motor areas for use in MOTOR CONTROL (Kemp and Powell 1971). New observations have led to the suggestion that basal ganglia loops are involved in a much more diverse range of behavior including MOTOR LEARNING and cognition. For example, Alexander, DeLong and Strick (1986) have proposed that basal ganglia output targeted at least five regions of the frontal lobe: two cortical areas concerned with skeletomotor and OCULOMOTOR CONTROL, and three regions of the prefrontal cortex involved in WORKING MEMORY, ATTENTION, and emotional behavior.
Subsequent experiments have supported this proposal and also suggested that basal ganglia-thalamocortical projections to the frontal lobe are topographically organized into discrete output channels (Hoover and Strick 1993; Middleton and Strick 1994). Furthermore, it is now apparent that basal ganglia output is directed to cortical areas outside the frontal lobe, including a region of the temporal lobe involved in visual processing (Middleton and Strick 1996a). Thus the anatomical substrate exists for basal ganglia output to influence multiple motor and nonmotor areas of the cerebral cortex. Consequently, current views of basal ganglia function emphasize the impact these subcortical nuclei may have on a broad spectrum of behavior.
Several lines of evidence implicate the basal ganglia in forms of "habit learning" that involve the creation of novel associations between stimuli and responses. For example, individuals with Parkinson's disease (PD) or Huntington"s disease (HD) have been shown to be impaired in the performance of tasks that depend on habit learning (Knowlton, Mangels, and Squire 1996; Knowlton et al. 1996). Both PD and HD arise from the degeneration of specific cell groups in the basal ganglia (the SNpc and striatum, respectively). Interestingly, SINGLE-NEURON RECORDING studies in monkeys have shown that "tonically active neurons" in the striatum change their firing properties as an association is built between a specific sensory stimulus and an appropriate motor response (Aosaki et al. 1994). These neurons are thought to be large (50-60 m) aspiny cholinergic interneurons. Similarly, some neurons in the SNpc are preferentially activated by appetitive rewards or stimuli that predict the occurrence of such rewards. Together, these striatal and nigral neurons may form part of the neural substrate underlying behavioral reinforcement (Schultz, Dayan, and Montague 1997).
Other forms of learning also appear to be influenced by the basal ganglia. Physiological studies have shown that portions of the striatum and pallidum are activated during the performance of tasks that require learning a sequence of movements (Jenkins et al. 1994; Mushiake and Strick 1995; Kermadi and Joseph 1995). Moreover, some patients with PD and HD are selectively impaired on motor learning tasks, but not on other forms of learning (Heindel et al. 1989). These observations suggest that the basal ganglia may play a critical role in what has been termed procedural or motor-skill learning.
There is also evidence to support the involvement of the basal ganglia in non-motor cognitive processes. First, some neurons in the basal ganglia display activity related to sensory and cognitive functions but not motor responses (Hikosaka and Wurtz 1983; Mushiake and Strick 1995; Brown, Desimone, and Mishkin 1996). Second, some individuals with PD and HD have striking cognitive and visual deficits, such as impaired recognition of faces and facial expressions, that actually precede the development of prominent motor symptoms (Jacobs, Shuren, and Heilman 1995a,b). Third, other patients with basal ganglia lesions exhibit profound cognitive, visual, and sensory disturbances. For example, lesions of the globus pallidus or SNpr have been reported to produce working memory deficits, obsessive-compulsive behavior, apathy, and visual hallucinations (Laplane et al. 1989; McKee et al. 1990). There is also growing evidence that alterations in the basal ganglia accompany disorders such as schizophrenia, depression, obsessive-compulsive disorder, Tourette"s syndrome, AUTISM, and attention deficit disorder (for references, see Middleton and Strick 1996b; Castellanos et al. 1996). Finally, the current animal model of PD uses high doses of a neurotoxin called MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) to reproduce the neuropathology and motor symptoms of this disorder with remarkable fidelity. However, chronic low-dose treatment of monkeys with this compound has been shown to cause cognitive and visual deficits, without gross motor impairments (Schneider and Pope-Coleman 1995).
Taken together, existing anatomical, physiological, and behavioral data suggest that the basal ganglia are not only involved in the control of movement, but also have the potential to influence diverse aspects of behavior. Future research will be needed to determine the full extent of the cerebral cortex influenced by basal ganglia output, the physiological consequences of this influence, and the functional operations performed by basal ganglia circuitry.
Alexander, G. E., M. R. DeLong, and P. L. Strick. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience 9:357-381.
Aosaki, T., H. Tsubokawa, A. Ishida, K. Watanabe, A. M. Graybiel, and M. Kimura. (1994). Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. Journal of Neuroscience 14:3969-3984.
Brown, V. J., R. Desimone, and M. Mishkin. (1996). Responses of cells in the tail of the caudate nucleus during visual discrimination learning. Journal of Neurophysiology 74:1083-1094.
Castellanos, F. X., J. N. Giedd, W. L. Marsh, S. D. Hamburger, A. C. Vaituzis, D. P. Dickstein, S. E. Sarfatti, Y. C Vauss, J. W. Snell, N. Lange, D. Kaysen, A. L. Krain, G. F. Ritchie, J. C. Rajapakse, and J. L. Rapoport. (1996). Quantitative brain magnetic resonance imaging in attention deficit hyperactivity disorder. Archives of General Psychiatry 53:607-616.
Heindel, W. C., D. P. Salmon, C. W. Shults, P. A. Walicke, and N. Butters. (1989). Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. Journal of Neuroscience 9:582-587.
Hikosaka, O., and R. H. Wurtz. (1983). Visual and oculomotor functions of monkey substantia nigra pars reticulata: 1. Relation of visual and auditory responses to saccades. Journal of Neurophysiology 49:1230-1253.
Hoover, J. E., and P. L. Strick. (1993). Multiple output channels in the basal ganglia. Science 259:819-821.
Jacobs, D. H., J. Shuren, and K. M. Heilman. (1995a). Impaired perception of facial identity and facial affect in Huntington's disease. Neurology 45:1217-1218.
Jacobs, D. H., J. Shuren, and K. M. Heilman. (1995b). Emotional facial imagery, perception, and expression in Parkinson's disease. Neurology 45:1696-1702.
Jenkins, I. H., D. J. Brooks, P. D. Nixon, R. S. Frackowiak, and R. E. Passingham. (1994). Motor sequence learning: A study with positron-emission tomography. Journal of Neuroscience 14:3775-3790.
Kemp, J. M., and T. P. S. Powell. (1971). The connexions of the striatum and globus pallidus: synthesis and speculation. Philosophical Transactions of the Royal Society of London, B262:441-457.
Kermadi, I., and J. P. Joseph. (1995). Activity in the caudate nucleus of monkey during spatial sequencing. Journal of Neurophysiology 74:911-933.
Knowlton, B. J., J. A. Mangels, and L. R. Squire. (1996). A neostriatal habit learning system in humans. Science 273:1399-1402.
Knowlton, B. J., L. R. Squire, J. S. Paulsen, N. R. Swerdlow, M. Swenson, and N. Butters. (1996). Dissociations within nondeclarative memory in Huntington's disease. Neuropsychology 10:538-548.
Laplane, D., M. Levasseur, B. Pillon, B. Dubois, M. Baulac, B. Mazoyer, S. Tran Dinh, G. Sette, F. Danze, and J. C. Baron. (1989). Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. Brain 112:699-725.
McKee, A. C., D. N. Levine, N. W. Kowall, and E. P. Richardson. (1990). Peduncular hallucinosis associated with isolated infarction of the substantia nigra pars reticulata. Annals of Neurology 27:500-504.
Middleton, F. A., and P. L. Strick. (1994). Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266:458-461.
Middleton, F. A., and P. L. Strick. (1996a). The temporal lobe is a target of output from the basal ganglia. Proceedings of the National Academy of Sciences, U.S.A. 93:8683-8687.
Middleton, F. A., and P.L. Strick. (1996b). Basal ganglia and cerebellar output influences non-motor function. Molecular Psychiatry 1:429-433.
Mushiake, H., and P.L. Strick. (1995). Pallidal neuron activity during sequential arm movements. Journal of Neurophysiology 74:2754-2758.
Parent, A., and L.-N. Hazrati. (1995). Functional anatomy of the basal ganglia: 1. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews 20:91-127.
Ragsdale, C. W., and A. M. Graybiel. (1981). The fronto-striatal projection in the cat and monkey and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Research 208:259-266.
Ringer, S. (1879). Notes of a postmortem examination on a case of athetosis. Practitioner 23: 161.
Schneider, J. S., and A. Pope-Coleman. (1995). Cognitive deficits precede motor deficits in a slowly progressing model of parkinsonism in the monkey. Neurodegeneration 4:245-255.
Schultz, W., P. Dayan, and P. R. Montague. (1997). A neural substrate of prediction and reward. Science 275:1593-1599.
Albin, R. L., A. B. Young, and J. B. Penney. (1989). The functional anatomy of basal ganglia disorders. Trends in Neuroscience 12:355-375.
Brown, L. L., J. S. Schneider, and T.I. Lidsky. (1997). Sensory and cognitive functions of the basal ganglia. Current Opinion in Neurobiology 7:157-163.
Carpenter, M. B., K. Nakano, and R. Kim. (1976). Nigrothalamic projections in the monkey demonstrated by autoradiographic technics. Journal of Comparative Neurology 165:401-416.
Cummings, J. L. (1993). Frontal-subcortical circuits and human behavior. Archives of Neurology 50:873-880.
DeLong, M. R. (1990). Primate models of movement disorders. Trends in Neuroscience 13:281-285.
DeVito, J. L., and M. E. Anderson. (1982). An autoradiographic study of efferent connections of the globus pallidus in Macaca mulatta. Experimental Brain Research 46:107-117.
Divac, I., H. E. Rosvold, and M. K. Swarcbart. (1967). Behavioral effects of selective ablation of the caudate nucleus. Journal of Comparative and Physiological Psychology 63:184-190.
Dubois, B., and B. Pillon. (1997). Cognitive deficits in Parkinson's disease. Journal of Neurology 244:2-8.
Eblen, F., and A. M. Graybiel. (1995). Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. Journal of Neuroscience 15:5999-6013.
Gerfen, C. R. (1984). The neostriatal mosaic: Compartmentalization of corticostriatal input and striatonigral output systems. Nature 311:461-464.
Goldman, P. S., and W. J. H. Nauta. (1977). An intricately patterned prefronto-caudate projection in the rhesus monkey. Journal of Comparative Neurology 171:369-386.
Graybiel, A. M. (1995). Building action repertoires: Memory and learning functions of the basal ganglia. Current Opinion in Neurobiology 5:733-741.
Ilinsky, I. A., M. Jouandet, and P. S. Goldman-Rakic. (1985). Organization of the nigrothalamocortical system in the rhesus monkey. Journal of Comparative Neurology 236:315-330.
Kim, R., K. Nakano, A. Jayarman, and M. B. Carpenter. (1976). Projections of the globus pallidus and adjacent structures: An autoradiographic study in the monkey. Journal of Comparative Neurology 169:263-290.
Lawrence, A. D., B. J. Sahakian, J. R. Hodges, A. E. Rosser, K. W. Lange, and T. W. Robbins. (1996). Executive and mnemonic functions in early Huntington's disease. Brain 119:1633-1645.
Nauta, W. J. H., and W. R. Mehler. (1966). Projections of the lentiform nucleus in the monkey. Brain Research 1:3-42.
Percheron, G., C. Francois, B. Talbi, J. Yelnik, and G. Fenelon. (1996). The primate motor thalamus. Brain Research Reviews 22:93-181.
Pillon, B., S. Ertle, B. Deweer, M. Sarazin, Y. Agid, and B. Dubois. (1996). Memory for spatial location is affected in Parkinson's disease. Neuropsychologica 34:77-85.
Saint-Cyr, J. A., L. G. Ungerleider, and R. Desimone. (1990). Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. Journal of Comparative Neurology 298:129-156.
Salmon, D. P., and N. Butters. (1995). Neurobiology of skill and habit learning. Current Opinion in Neurobiology 5:184-190.
Schultz, W. (1997). Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology 7:191-197.
Selemon, L. D., and P. S. Goldman-Rakic. (1985). Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. Journal of Neuroscience 5:776-794.
Strub, R. L. (1989). Frontal lobe syndrome in a patient with bilateral globus pallidus lesions. Archives of Neurology 46:1024-1027.
Taylor, A. E., and J. A. Saint-Cyr. (1995). The neuropsychology of Parkinson's disease. Brain and Cognition 23:281-296.
Wise, S. P., E. A. Murray, and C. R. Gerfen. (1996). The frontal cortex-basal ganglia system in primates. Critical Reviews in Neurobiology 10:317-356 .