Figure 1
Working memory, as defined by cognitive psychologists, refers to "a system for the temporary holding and manipulation of information during the performance of a range of cognitive tasks such as comprehension, learning and reasoning" (Baddeley 1986). The adjective "working" is a critical part of the definition, emphasizing as it does the processing of information and not its particular content. Working memory is characterized by its limited storage capacity and rapid turnover and is differentiated from the larger capacity and archival memory system traditionally defined as long-term memory. The origin of the term "working memory" is difficult to trace. It was used by Miller, Galanter, and Pribram in their 1960 book, Plans and the Structure of Behavior, to describe the functions of the frontal lobe: "This most forward portion of the primate frontal lobe appears to us to serve as a "working memory" where plans . . . can be retained temporarily when they are being formed, or transformed, or executed." Metaphors for working memory include "blackboard of the mind" (Reddy 1980); "mental sketch-pad" (Baddeley 1986), and "on-line memory" (Goldman- Rakic 1987). When we listen to human speech, we are using working memory to hold the segments of sentences "on-line" millisecond by millisecond. We employ working memory to carry forward, in real time, the subject of a sentence and associate it with verbs and objects in order to comprehend the sense and meaning of sentences. When we perform a mental arithmetic problem, recall a phone number, plan a hand of bridge or a chess move, or follow a verbal instruction, we use working memory. In fact it is difficult to think of a cognitive function that does not engage the working-memory systems of the brain. A number of different models have been proposed regarding the functional architecture of human cognition (see WORKING MEMORY).
The study of short-term memory in nonhuman primates can be traced at least as far back as the seminal work of Jacobsen and Fulton which showed a dependence of delayed-response performance on the dorsolateral prefrontal areas of the primate frontal lobe (Jacobsen 1936). Delayed-response tasks are those in which a brief delay is introduced between the presentation of a stimulus (usually denoting either a location or the identity of an object) and the response that is required to indicate that the stimulus has been recalled. It is important to point out, that, as studied in the 1930s and forward, delayed-response tasks were considered tests primarily of "immediate memory." This terminology denoted passive or short-term storage and did not embody the notion of processing or the linkage between storage and processing that are central concepts in the study of working memory (Baddeley 1986). For commonly used tasks of the delayed-response "family," little or no processing is in fact required. However, there is much evidence to indicate that the storage and processing functions within working memory are carried out by the same cells and circuits and that the study of the machinery for storage is an essential step in understanding the processes carried out by those cells and circuits.

Figure 1
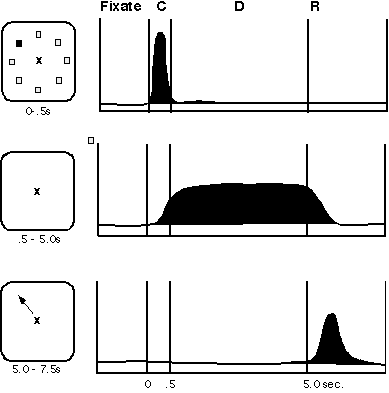
Working memory is studied in humans mainly by the use of behavioral paradigms, most recently in conjunction with brain imaging. However, the physiological and circuit underpinnings of this process have come primarily from anatomical, physiological, and behavioral research in nonhuman primates. A cellular basis for working memory has emerged from the study of activity in single neurons recorded from the prefrontal cortex of monkeys that have been trained to perform delayed response tasks (Fuster and Alexander 1971; Kubota and Niki 1971; Goldman-Rakic, Lidow, and Gallager 1990). In the traditional type of spatial delayed-response task, a monkey observes an experimenter place a food morsel in one of two food wells, each of which is immediately covered by identical cards. A screen is lowered between the test tray to prevent the monkey from immediately displacing the card to reveal the hidden treat. After several seconds, the screen is raised and the animal is allowed to select one of the food wells. The imposed delay forces the monkey to base its choice on the memory of the location in which the food was placed before the delay, that is, the choice is memory-guided. Monkeys with lesions of their dorsolateral prefrontal cortices are unable to make correct choices but instead respond at random (Goldman-Rakic 1987; Fuster 1989). Physiological studies have revealed that the activity of prefrontal neurons correlates with events in the delayed-response tasks, that is, some neurons respond during the placement of the food stimulus, others respond during the delay period, and still other neurons respond at the time of the response (figure 1). Many neurons are combinatorial, having cue, delay, and response-related responses. The activity profiles of prefrontal neurons are thus strikingly related to the subfunctions of sensory registration, memory, and motor control, respectively. As the cue, delay, and response-related neurons are necessarily activated in sequence rather than simultaneously, these neurons mediate the real time events in working memory.
The neurons that respond in the delay period are of particular interest because they exhibit sustained activity for many seconds in the absence of any stimulus, a property that is not observed in primary sensory areas of the brain. Using an oculomotor paradigm which requires the monkey to fixate while small stimuli are presented in various locations in the visual field, it became possible to show that these prefrontal neurons have "memory fields," defined as maximal firing of a neuron to the representation of a target in one or a few locations of the visual field (Funahashi, Bruce, and Goldman-Rakic 1989). For example, a neuron's activity may rise sharply after a stimulus is presented briefly at its "preferred" (e.g., 270°) position, then remain tonically active during a 3 to 5 sec delay (in the absence of the stimulus), and then return to baseline activation abruptly at the end of delay when the response is initiated (as displayed in figure 2). Importantly, such activation occurs every time the animal has to remember the same location but not when the animal is remembering targets presented at other "nonpreferred" locations (e.g., 135°, 180°, 225°). An additional important feature of many prefrontal neurons is that while their rate of firing in the delay period is enhanced for one target location, it may be inhibited during the delay on trials with target stimuli of opponent polarity; such a pattern of activity indicates that some prefrontal neurons have "opponent memory fields." This functional distinction provides a valuable clue to how the neural circuitry subserving working memory might be organized. In particular, it points to the role of neural inhibition in sculpting the memory field of these neurons. Inhibition is provided by interneurons in the immediate vicinity of the pyramidal neurons which express memory fields and with the capacity to transmit the information to other areas of the brain. Finally, neuronal activity in the delay period appears essential to correct recall of the preceding stimulus; errors are invariably made on trials in which neurons with memory fields fail to maintain their activation during the delay period (Funahashi et al. 1989).
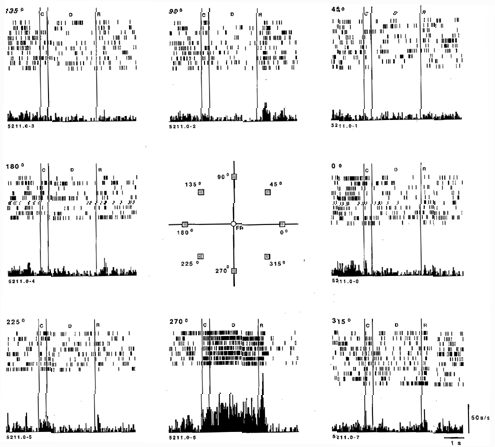
Figure 2
From CAJAL on, it has been appreciated that several types of interneurons populate the CEREBRAL CORTEX and interact with pyramidal cells. The overwhelming majority of interneurons utilize the inhibitory neurotransmitter, g-aminobutyric acid (GABA), whereas pyramidal cells use the excitatory amino acid glutamate as their neurotransmitter. Recent evidence indicates that pyramidal-nonpyramidal interactions are critical to the formation of memory fields in prefrontal cortex just as they may play a role in establishing the orientation specificity of primary visual neurons. Wilson, O'Scalaidhe, and Goldman-Rakic (1994) have succeeded in classifying prefrontal neurons as interneurons or pyramidal neurons based on their firing rates in vivo, that is, as monkeys performed the oculomotor delayed response task. This study showed that interneurons, like pyramidal neurons, express directional preferences and the patterns of activity expressed by closely adjacent pyramidal and nonpyramidal neurons are often inverse, such that as a nonpyramidal neuron increases its rate of discharge, a nearby pyramidal neuron decreases its rate. Current studies are examining in more detail the nature of interactions between interneurons and pyramidal neurons engaged in the working memory process. At present, it is clear that the mechanism of disinhibition is a process that plays a powerful role in the construction of a prefrontal neuron's memory field. Further, it is becoming clear that modulatory NEUROTRANSMITTERS such as dopamine and serotonin modulate the activity of and interactions between pyramidal and nonpyramidal cells.
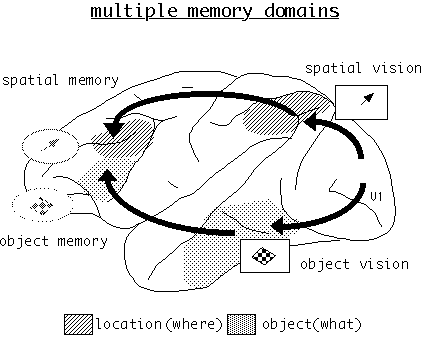
Figure 3
Spatial and feature working memory mechanisms of prefrontal cortex are dissociable at the behavioral, cellular, and areal levels of functional architecture (figure 3). In nonhuman primates, lesions of the dorsolateral prefrontal cortex produce deficits on spatial working-memory tasks but not on object working-memory tasks while, conversely, lesions of the inferior convexity of the prefrontal cortex produce deficits on object-memory tasks but not on spatial tasks (Goldman-Rakic 1987; Mishkin 1964). Consistent with lesion and behavior results, neurons located in dorsolateral prefrontal cortex (areas 46 and 8) code visual-spatial memoranda, while those that code simple, complex, or categorical features of stimuli are located in the inferior convexity (areas 12 and 45; Wilson, O'Scalaidhe, and Goldman-Rakic 1993; O'Scalaidhe, Wilson, and Goldman-Rakic 1997). Moreover, individual neurons that are engaged by the memory of an object rarely, if ever, code their peripheral location (Wilson et al. 1993). Physiologically guided injections of pathway tracers in the spatial and object-memory centers have shown them to be connected to the appropriate visual centers in the parietal (Cavada and Goldman-Rakic 1989) and temporal lobes, respectively (Webster, Bachevalier, and Ungerleider 1994; Bates et al. 1994).
Working memory is considered a major component of the machinery of executive function and it is not surprising that POSITRON EMISSION TOMOGRAPHY (PET) and functional MAGNETIC RESONANCE IMAGING (fMRI) studies in human subjects have focused on this function. Recent studies in healthy human subjects, for example, have shown that the middle frontal gyrus, the region corresponding to the dorsolateral areas studied in macaque monkeys, is activated when human subjects carry out analogous spatial working-memory tasks (McCarthy et al. 1994; Jonides et al. 1993). Inferior regions of the dorsolateral prefrontal cortex are activated for verbal and other nonspatial working-memory functions (e.g., Frith et al. 1991; Courtney et al. 1996; Demb et al. 1995). All of these results speak to the modular organization of working -memory systems in the prefrontal cortex, with each working-memory "domain" associated with an informationally constrained sensory-processing stream.
Working-memory capacity in human subjects can be assessed by numerous tasks designed by cognitive psychologists using visual or auditory stimuli and verbal, spatial, and object vision. Many of these tasks are formally similar to those used with nonhuman primates, assuring the generalizability of results from the nonhuman species to humans. fMRI and PET have been used to examine changes in blood flow or metabolic activity in the cerebral cortex of normal human subjects performing a wide variety of working- memory tasks. The human prefrontal areas that are invariably activated under these behavioral conditions are dissociable from the areas activated by sensorimotor components of the tasks examined (Cohen et al. 1994; Courtney et al. 1996; Sweeney et al. 1996) as they are in nonhuman primates (for review, see Goldman-Rakic 1987; also Goldman et al. 1971).
Conversely, prefrontal areas display depressed blood flow or metabolism in patients with schizophrenia (e.g., Weinberger, Berman, and Zec 1986), depression (e.g., Buchsbaum, Debisi, and Holcomb 1984) and other conditions of impaired affect or cognition. As might be expected if working memory were essential to executive function, working-memory deficits and correlated prefrontal dysfunction have been demonstrated in schizophrenics (e.g., Weinberger et al. 1986; Fukushima et al. 1988; Park and Holzman 1992), in Parkinson patients (e.g., Gotham, Brown, and Marsden 1988; Levin, Labre, and Weiner 1989), and in age-related memory decline (e.g., Salthouse 1991) -- neuropathological conditions in which impairments of higher cortical processing are expressed. Prefrontal cortical volume is reduced in schizophrenia, (e.g., Zipursky et al. 1992) and cellular changes (e.g., Benes et al. 1991; Selemon, Rajkowska, and Goldman-Rakic 1995) have also been observed in prefrontal areas in this disorder. On the strength of these and other findings, Goldman-Rakic has proposed that working memory dysfunction may be the core functional deficit underlying thought disorder in schizophrenia (Goldman-Rakic 1987, 1991). It has now become clear that schizophrenic patients are also impaired in tasks requiring working memory. Park and Holzman (1992) have demonstrated that patients with schizophrenia express delay-dependent errors on a modified spatial oculomotor delayed-response paradigm nearly identical to that used in the single-unit studies described above. Early evidence indicates that relatives of patients perform more poorly on spatial working-memory tasks than unrelated control subjects (Park, Holzman, and Goldman-Rakic 1995). Auditory working memory is also severely impaired in schizophrenic patients (Gold et al. 1997; Saykin et al. 1991). These and many other studies are laying the basis for a comprehensive analysis of psychiatric disorders in terms of working memory and the prefrontal mechanisms upon which it greatly depends.
Baddeley, A. (1986). Working Memory. London: Oxford University Press.
Bates, J. F., F. A. W. Wilson, S. P. O'Scalaidhe, and P. S. Goldman-Rakic. (1994). Area TE connections with inferior prefrontal regions responsive to complex objects and faces. Soc. Neurosci. Abstr. 20: 1054.
Benes, F. M., J. McSparren, E. D. Bird, J. P. San Giovanni, and S. L. Vincent. (1991). Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch. Gen. Psychiatry 48:996-1001.
Buchsbaum, M. S., L. E. DeLisi, and H. H. Holcomb. (1984). Anteroposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Arch. Gen. Psychiatry 41:1159-1166.
Cavada, C., and P. S. Goldman-Rakic. (1989). Posterior parietal cortex in rhesus monkey: 2. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J. Comp. Neurol 287:422-445.
Cohen, J. D., S. D. Forman, T. S. Braver, B. J. Casey, D. Servan-Schreiber, and D. C. Noll. (1994). Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum. Brain Mapping 1:293-304.
Courtney, S. M., L. G. Ungerleider, K. Keil, and J. V. Haxby. (1996). Object and spatial visual working memory activate separate neural systems in human cortex. Cereb. Cortex 6:39-49.
Demb, J. B., J. E. Desmond, A. D. Wagner, C. J. Vaidya, G. H. Glover, and J. D. E. Gabrieli. (1995). Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficult and process specificity. J. Neurosci. 15:5870-5878.
Frith, C. D., K. Friston, P. F. Liddle, and R. S. J. Frackowiak. (1991). Willed action and the prefrontal cortex in man. Proc. R. Soc. Lond. B. Biol. Sci. 244:241-246.
Fukushima, J., K. Fukushima, T. Chiba, S. Tanaka, I. Yamashita, and M. Kato. (1988). Disturbances of voluntary control of saccadic eye movements in schizophrenic patients. Biol. Psychiatry 23:670-677.
Funahashi, S., C. J. Bruce, and P. S. Goldman-Rakic. (1989). Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol 61:331-349.
Fuster, J. M. (1989). The Prefrontal Cortex. 2nd ed. New York: Raven Press.
Fuster, J. M., and G. E. Alexander. (1971). Neuron activity related to short-term memory. Science 173:652-654.
Gold, J. M., C. Carpenter, C. Randolph, T. E. Goldberg, and D. R. Weinberger. (1997). Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch. Gen. Psychiatry 54:159-165.
Goldman, P. S., H. E. Rosvold, B. Vest, and T. W. Galkin. (1971). Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J. Comp. Physiol. Psychol. 77: 2l2-220.
Goldman-Rakic, P. S. (1987). Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In F. Plum and V. Mountcastle, Eds., Handbook of Physiology. Bethesda, MD: American Physiological Society, pp. 373-417.
Goldman-Rakic, P. S. (1991). Prefrontal cortical dysfunction in schizophrenia: The relevance of working memory. In B. J. Carroll and J. E. Barrett, Eds., Psychopathology and the Brain. New York: Raven Press, pp. 1-23.
Goldman-Rakic, P. S., M. S. Lidow, and D. W. Gallager. (1990). Overlap of dopaminergic, adrenergic, and serotonergic receptors and complementarity of their subtypes in primate prefrontal cortex. J. Neurosci. 10:2125-2138.
Gotham, A. M., R. G. Brown, and C. P. Marsden. (1988). "Frontal" cognitive function in patients with Parkinson's disease "on" and "off" levodopa. Brain 111:299-321.
Jacobsen, C. F. (1936). Studies of cerebral function in primates. Comp. Psychol. Mono. 13:1-68.
Jonides, J., E. E. Smith, R. A. Koeppe, E. Awh, S. Minoshima, and M. A. Mintun. (1993). Spatial working memory in humans as revealed by PET. Nature 363:623-625.
Kubota, K., and H. Niki. (1971). Prefrontal cortical unit activity and delayed cortical unit activity and delayed alternation performance in monkeys. J. Neurophysiol. 34:337-347.
Levin, B. E., M. M. Labre, and W. J. Weiner. (1989). Cognitive impairments associated with early Parkinson's disease. Neurology 39:557-561.
McCarthy, G., A. M. Blamire, A. Puce, A. C. Nobre, G. Bloch, F. Hyder, P. S. Goldman-Rakic, and R. G. Shulman. (1994). Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc. Natl. Acad. Sci. USA 91:8690-8694.
Miller, G. A., E. H. Galanter, and K. H. Pribram. (1960). Plans and Structure of Behavior. New York: Holt.
Mishkin, M. (1964). Perseveration of central sets after frontal lesions in monkeys. In J. M. Warren and K. Akert, Eds., The Frontal Granular Cortex and Behavior. New York: McGraw-Hill, p. 219.
O'Scalaidhe, S. P., F. A. W. Wilson, and P. S. Goldman-Rakic. (1997). Area segregation of face-processing neurons in prefrontal cortex. Science 278:1135-1138.
Park, S. and P. S. Holzman. (1992). Schizophrenics show spatial working memory deficits. Arch. Gen. Psychiatry 49:975-982.
Park, S., P. S. Holzman, and P. S. Goldman-Rakic. (1995). Spatial working memory deficits in the relatives of schizophrenic patients. Arch. Gen. Psychiatry 52:821-828.
Reddy, E. R. (1980). Machine models of speech perception. In R. A. Cole, Ed., Perception and Production of Human Speech. Hillsdale, NJ: Erlbaum pp. 215-242.
Salthouse, T. A. (1991). Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychological Sciences 2:170-183.
Saykin, A. J., R. C. Gur, R. E. Gur, P. D. Mozley, L. H. Mozley, S. M. Resnick, B. Kester, and P. Stafiniak. (1991). Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Arch. Gen. Psychiatry 48:618-624.
Selemon, L. D., G. Rajkowska, and P. S. Goldman-Rakic. (1995). Abnormally high neuronal density in two widespread areas of the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry 52:805-818.
Sweeney, J. A., M. A. Mintun, B. S. Kwee, M. B. Wiseman, D. L. Brown, D. R. Rosenberg, and J. R. Carl. (1996). A positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J. Neurophysiol. 75:454-468.
Webster, M. J., J. Bachevalier, and L. G. Ungerleider. (1994). Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb. Cortex 4:470-483.
Weinberger, D. R., K. F. Berman, and R. F. Zec. (1986). Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I: Regional cerebral blood flow (rCBF) evidence. Arch. Gen. Psychiatry 43:114-125.
Wilson, F. A. W., S. P. O'Scalaidhe, and P. S. Goldman-Rakic. (1993). Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260:1955-1958.
Wilson, F. A. W., S. P. O'Scalaidhe, and P. S. Goldman-Rakic. (1994). Functional synergism between putative g- aminobutyrate- containing neurons and pyramidal neurons in prefrontal cortex. Proc. Natl. Acad. Sci. USA 91:4009-4013.
Zipursky, R. B., K. O. Lim, E. V. Sullivan, B. W. Brown, and A. Pfefferbaum. (1992). Widespread cerebral gray matter volume deficits in schizophrenia. Arch. Gen. Psychiatry 42:195-205.