Sleep is a behavioral adaptation of vertebrate animals with much to teach the cognitive scientist about the relationship of mind to brain. In no other behavioral state are the differences from waking psychology so profound or so clearly tied to the underlying changes in neurophysiology. It is this psychophysiological concomitance that will be emphasized in the account given here of the natural history and neurobiology of sleep.
As a behavior, sleep is characterized by (1) a recumbent posture with varying degrees of relaxation of the skeletal musculature; (2) an increase in the threshold of response to sensory stimuli; and (3) a characteristic set of electrographic signs. From an evolutionary point of view sleep is clearly a strategy for energy conservation and for protection from predators since all animals sleep at times and in places that confer a benefit in one or both of these domains. Sleep is distinguished from simple rest, from torpor, and from anesthetic or traumatic unresponsiveness by its active and distinctive brain mechanisms of induction and maintenance, as well as by its ready reversibility.
As a function of the circadian rhythm generated by the suprachiasmatic nucleus of the hypothalamus, all vertebrate animals show prominent rest-activity cycles with an endogenous period of about one day. Corresponding to the increased complexity of the supervening thalamocortical brain and the greater sophistication of their thermoregulatory capacity, the vertebrate mammals link active sleep control mechanisms in the lower brain stem to the circadian cycle. The result is a stereotyped sequence of events that is coordinated throughout the brain so as to alter every aspect of cognition in a dramatic and sometimes paradoxical fashion. The most surprising aspect of this automatic sequence of events is the regular recurrence of periods of brain activation and rapid eye movement (REM) in sleep that is associated in humans with hallucinoid DREAMING.
Waking, with all of its cognitive components, is an actively maintained brain state in which the thalamocortical circuitry is kept open and receptive to information from within and without by the depolarization of reticular thalamic neurons by cholinergic and aminergic modulatory elements of the brain stem. This activation, together with its specific neuromodulatory effects, renders the forebrain capable of sensation, perception, ATTENTION, orientation, emotion, and stimulus evaluation in terms of past experience, and deliberate action. Whenever the brain stem neuromodulatory influence declines to a critical level, the thalamocortical system tends to oscillate, produ-cing its own endogenous rhythm of electroencephalographic (EEG) spindles and slow waves which are incompatible with waking conscious experience because the inputs to and outputs from the cortex are blocked and intracortical communication is preempted. Cognitive function is thus progressively obtunded as this progressive brain deactivation proceeds in nocturnal sleep. This is reflected by the shift from stage I to stage IV of so-called non-REM (NREM) sleep from the depths of which human subjects are very difficult to rouse. Even when verbally responsive they may then show marked sleep inertia with persistent slow waves in the EEG and an inability to perform even trivially simple cognitive tasks such as serial seven subtraction. This sleep inertia process is greatest in the first two NREM cycles of the night and is intensified in postdeprivation recovery sleep.

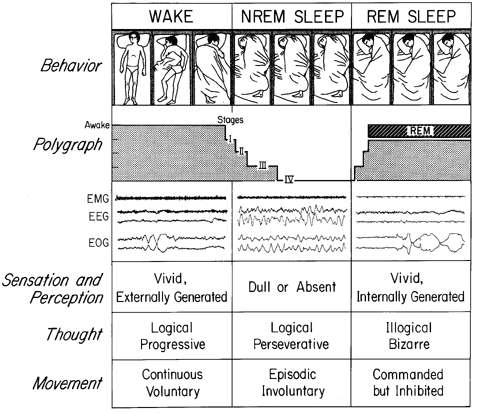
Figure 1 Sleep cycle schematic. The states of waking, NREM, and REM sleep have behavioral, polygraphic, and psychological manifestations that are depicted here. In the behavioral channel, posture shifts -- detectable by time-lapse photography or video -- can be seen to occur during waking and in concert with phase changes of the sleep cycle. Two different mechanisms account for sleep immobility: disfacilitation (during stages I - IV of NREM sleep) and inhibition (during REM sleep). In dreams, we imagine that we move, but we do not. The sequence of these stages is schematically represented in the polygraph channel and sample tracings are also shown. Three variables are used to distinguish these states: the electromyogram (EMG), which is highest in waking, intermediate in NREM sleep, and lowest in REM sleep; and the electroencephalogram (EEG) and electrooculogram (EOG), which are both activated in waking and REM sleep, and inactivated in NREM sleep. Each sample record is about 20 sec long. Other subjective and objective state variables are described in the three lower channels. (From J. A. Hobson and M. Steriade (1986), Neuronal basis of behavioral state control. In V. Mountcastle and F. E. Bloom, Eds., Handbook of Physiology: The Nervous System. Vol. 4, pp. 701-823.)
Recent POSITRON EMISSION TOMOGRAPHY (PET) studies of human NREM sleep have revealed decreased blood flow in the brain stem reticular formation, the subthalamus, and in frontal cortical regions denoting the massive deactivation of these structures in NREM sleep. Animal studies further confirm this deactivation process at the level of individual neurons, many of which decrease their rate of firing by as much as 50 percent. There is also a 50 percent decline in the output of the wake state neuromodulatory chemicals acetylcholine, norepinephrine, and serotonin. It is for all these reasons that the NREM sleeping brain is such a poor cognitive instrument and it is the carryover of these effects into subsequent waking that so severely impairs problem-solving behavior upon arousal from deep NREM sleep. That this cognitive impairment may nonetheless be beneficial is suggested by the finding that complex DECISION MAKING is more efficient during waking that follows nights with uninterrupted NREM sleep.
After sixty to seventy minutes of deep NREM sleep subjects show a reversal of these oscillatory EEG patterns and the brain spontaneously reactivates. Together with the EEG desynchronization is an activation of the upper brain's motor systems signaled by flurries of rapid saccadic movements of the eyes (the so-called REMs). However, the output of most of these signals in behavior is blocked by strong inhibition of final common path motor neurons. Postural tone is obliterated and signaled by the complete obliteration of electromyographic activity. Awakening subjects from REM sleep is much easier, they perform most tasks better, and they commonly give longer, more detailed dream reports than following NREM awakenings. Recent PET studies of human REM sleep indicate increased activation (compared even to waking) of the pontine brain stem, the basolateral forebrain, and especially the limbic subcortical and paralimbic cortical structures, while the more dorsofrontal cortical areas remain inactivated. This finding has a strong bearing on the strongly emotional character and the bizarreness of human dreaming because it indicates a bottom-up activation of the limbic brain with the release of emotions and related memories which must be integrated in conscious experience without the benefit of executive cortical guidance. Cellular-level studies in animals confirm the activation inferences of human PET studies for the pontine brain stem and other subcortical areas. In addition they describe a dissociation between cholinergic neuromodulation (which equals or exceeds that of waking) and noradrenergic and serotonergic neuromodulation which falls to near-zero levels.
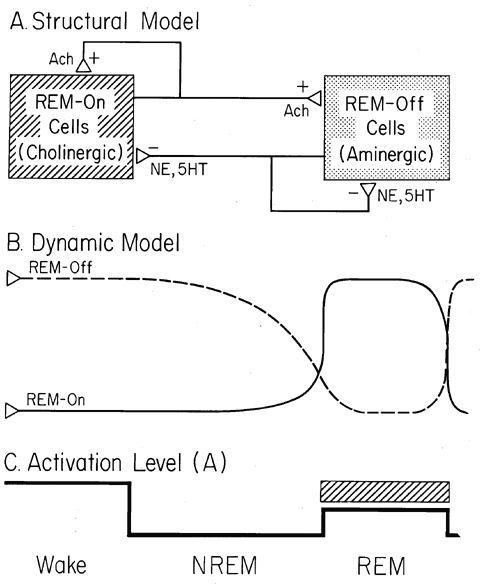
Figure 2 Reciprocal Interaction. (A) Structural model. REM-on cells of the pontine reticular formation are cholinoceptively excited and/or cholinergically excitatory (ACH+) at their synaptic endings (open boxes). Pontine REM-off cells are noradrenergically (NE) or serotonergically (5HT) inhibitory (-) at their synapses (filled boxes). (B) Dynamic model. During waking the pontine aminergic (filled box) system is tonically activated and inhibits the pontine cholinergic (open box) system. During NREM sleep aminergic inhibition gradually wanes and cholinergic excitation reciprocally waxes. At REM sleep onset aminergic inhibition is shut off and cholinergic excitation reaches its high point. (C) Activation level (A). As a consequence of the interplay of the neuronal systems shown in A and B, the net activation level of the brain (A) is at equally high levels in waking and REM sleep and at about half this peak level in NREM sleep.
These paradoxical findings show that the brain activation patterns of waking and REM sleep are actually quite different even though they are indistinguishable from an EEG point of view. They also shed light on the differences in cognition, especially the disorientation, the defective reasoning and judgment, and the poor memory during and after dreaming.
Subjects who are deprived of either NREM or REM sleep (or both) show progressively impaired cognitive capacities which may progress to psychotic disorganization if they can be kept awake despite the marked increase in their intrinsic drive to sleep. This finding indicates that sleep confers critical benefits to the brain and to cognitive capability. These benefits are yet to be fully elucidated but may relate to the dramatic alterations in neuromodulatory balance alluded to above. From a commonsense point of view, the hypothesis crying out for critical test is that sleep benefits cognition not only by cerebral energy conservation but also by a more specific and profound rest of the very chemical systems most critical to wake state cognition.
One of the most attractive hypotheses regarding the cognitive benefit of sleep is that it enhances LEARNING and MEMORY. Many experiments have shown increases in sleep to be associated with the mastery of learning tasks in both animal models and human subjects. Some of these increases are immediate while others are delayed. While most of these studies have emphasized REM sleep, others have presented evidence for a two-stage process with NREM sleep serving to iterate recently learned material followed by its consolidation in REM. This theory is congruent with the differential subjective experience of the two states of sleep: during NREM mental activity tends to be a perseverative and nonprogressive rumination regarding recent events; in contrast, REM sleep mentation melds both recent and remote memories in bizarre scenarios that are often associated with strong, usually unpleasant, emotion, suggesting the possibility that emotional salience is a feature of the consolidation process. The specification of these hypotheses in cellular and molecular terms presents cognitive neuroscience with one of its central challenges. Because so much is known about the neuromodulatory underpinnings of the waking and NREM and REM sleeping states, a unique opportunity exists to unite neurobiological sleep research with classic experimental psychology, solving a central problem of both fields simultaneously.
That the cognitive and more general energetic benefits of sleep may have a unitary underlying mechanism is suggested by animal experiments which have consistently demonstrated reciprocal impairment (by deprivation) and enhancement (by normal or recovery sleep) of both thermoregulatory and cognitive functions. So critical is sleep to the life of the mind and the body that its loss is at first deleterious and later ultimately fatal to both.
Braun, A. R., T. J. Balkin, N. J. Wesensten, R. E. Carson, M. Varga, P. Baldwin, S. Selbie, G. Belenky, and P. Herscovitch. (1997). Regional cerebral blood flow throughout the sleep-wake cycle. Brain 120:1173-1197.
Hennevin, E., B. Hars, C. Maho, and C. Bloch. (1995). Processing of learned information in paradoxical sleep: Relevance for memory. Behavioural Brain Research 69:125-135.
Hobson, J. A., R. W. McCarley, and P. W. Wyzinki. (1975). Sleep cycle oscillation: Reciprocal discharge by two brainstem neuronal groups. Science 189:55-58.
Hobson, J. A., R. Lydic, and H. Baghdoyan. (1986). Evolving concepts of sleep cycle generation: From brain centers to neuronal populations. Behav. Brain Sci. 9:371-448.
Hobson, J. A., and M. Steriade. (1986). The neuronal basis of behavioral state control. In F. E. Bloom, Ed., Handbook of Physiology -- The Nervous System, vol. 4. Bethesda, MD: American Physiological Society, pp. 701-823.
Hobson, J. A., and R. Stickgold. (1995). Sleep, the beloved teacher. Current Biology 5:35-36.
Maquet, P., J. M. Peters, J. Aerts, G. Delfiore, C. Degueldre, A. Luxen, and G. Franck. (1996). Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature 383: 163.
McCarley, R. W., and J. A. Hobson. (1975). Neuronal excitability modulations over the sleep cycle: A structured and mathematical model. Science 189:58-60.
Nofzinger, E. A., M. A. Mintun, M. B. Wiseman, D. Kupfer, and A. Y. Moore. (1997). Forebrain activation in REM sleep: An FDG PET study. Brain Res. 770:192-201.
Smith, C. (1996). Sleep states, memory processes, and synaptic plasticity. Behavioural Brain Research 78:49-56.
Steriade, M., and R. W. McCarley. (1990). Brainstem Control of Wakefulness and Sleep. New York: Plenum Press .