
Mental activity does not cease at the onset of sleep. Current scientific evidence suggests instead that it is virtually continuous throughout sleep but that its level of intensity and its formal characteristics change as the brain changes its state with the periodic recurrence of rapid eye movement (REM) and non-REM (NREM) sleep phases.

Figure 1 The Activation-Synthesis model. Systems and synaptic model. As a result of disinhibition caused by cessation of aminergic neuronal firing, brainstem reticular systems autoactivate. Their outputs have effects including depolarization of afferent terminals causing phasic presynaptic inhibition and blockade of external stimuli, especially during the bursts of REM, and postsynaptic hyperpolarization causing tonic inhibition of motorneurons that effectively counteract concomitant motor commands so that somatic movement is blocked. Only the oculomotor commands are read out as eye movements because these motorneurons are not inhibited. The forebrain, activated by the reticular formation and also aminergically disinhibited, receives efferent copy or corollary discharge information about somatic motor and oculomotor commands from which it may synthesize such internally generated perceptions as visual imagery and the sensation of movement, both of which typify dream mentation. The forebrain may, in turn, generate its own motor commands that help to perpetuate the process via positive feedback to the reticular formation.
Until the discovery of REM sleep by Eugene Aserinsky and Nathaniel Kleitman in 1953, interest in the psychology of dreaming was restricted to speculative accounts of its distinctive phenomenology that were linked to schematic efforts to interpret dream content. The best known example of this kind of theorizing is the psychoanalytic model of Sigmund FREUD, which held that dream bizarreness was the result of the mind's effort to disguise and censor unconscious wishes released in sleep that in their unaltered form would overwhelm the mind and cause awakening. The discovery of the association of dreaming with REM sleep allowed a quite different approach. Emphasis suddenly shifted from the attempt to analyze the content to an attempt to explain the formal aspects of the distinctive phenomenology in terms of underlying brain activity.
This article gives a summary of how the cellular and molecular changes in the brain which distinguish waking, NREM and REM sleep can be used to account for the concomitant shifts in mental state that result in the shift in consciousness from waking to dreaming. (See also SLEEP for relevant background information.)
Whether subjects are aroused from sleep in a laboratory setting or awaken spontaneously at home, they give reports of preawakening mental experience that are quite different if their brain state is REM than if it is non-REM. REM-sleep dream reports are seven times longer and are far more likely to describe formed sensory perceptions and vivid visual images than are the reports of NREM dreams, which tend to be more thoughtlike and dull. REM sleep reports are also far more likely to be animated, with descriptions of walking, running, playing sports, or even flying. Finally, the REM-sleep dream scenarios are accompanied by strong emotions such as anxiety, elation, and anger, all of which bear a close relationship to details of the plot.
These formal features of dreaming correlate well with changes in the activation level of the brain as measured by the degree of low-voltage, high-frequency power in the sleep electroencephalogram, and they are negatively correlated with high voltage, slow EEG patterns. Because the high-voltage, slow-wave activity of NREM sleep is most intense and prolonged in the first half of the night, reports from awakenings performed then are more likely to show differences from REM reports than are those from the second half of the night. Brain activation is therefore an easily understandable determinant of dream length and visual intensity. Dreamlike mentation may also emerge at sleep onset when the brain activation level is just beginning to fall. Sleep-onset dreaming is likely to be evanescent and fragmentary, with less vivid imagery, less strong emotion, and a less well developed story line than in REM-sleep dreaming.
Collaborating with the still high activation level to produce sleep-onset dreaming is the rapidly rising threshold to external sensory stimulation. This factor allows internal stimuli to dominate the brain. In REM sleep internal stimuli also protect the brain from external sensory influence. If the stimulus level is raised to sufficiently high levels, external information can be incorporated into dream plots, but the critical window for such incorporation is narrow and external stimuli more commonly interrupt dreaming by causing awakening. When dreams are interrupted in this way, recall of dreaming is markedly enhanced to levels as high as 95 percent if the subject is aroused from REM sleep during a cluster of rapid eye movements.
The strong correlation between dreaming and REM sleep has encouraged attempts to model the brain basis of dreaming at the cellular and molecular level. The activation synthesis hypothesis, first put forward in 1977, ascribed dreaming to activation of the brain in REM sleep by a well-specified pontine brain stem mechanism.
Such distinctive aspects of dream mentation as vivid visual hallucinations, a constant sense of movement, and strong emotion were ascribed to internal stimulation of visual, motor, and limbic regions of the upper brain by signals of brain stem origin. The bizarreness of dream cognition, with its characteristic instability of time, place, and person, was thought to be due to the chaotic nature of the autoactivation process and to the failure of short-term memory caused by the chemical changes in REM described by the reciprocal interaction model of brain state control first advanced in 1975.
Using microelectrode recording techniques to sample individual cell activity during natural sleep and waking in animal models, it has been possible to show that the neuromodulatory systems of the brain stem behave very differently in waking and REM sleep. These differences help to account for the distinctive psychological features of dreaming, especially the bizarreness and recent memory loss. During waking, cells of the noradrenergic locus coeruleus and the serotonergic raphe nuclei are tonically active, but in REM they are shut off. This means that the activated brain of REM is aminergically demodulated so that it cannot process information in the same way as it does in waking. Dream bizarreness and dream amnesia are both the result of this neuromodulatory defect. Compounding this difference, the pontine cholinergic neurones become reciprocally activated in REM, and their intense phasic activity conveys eye movement-related information to the visual sensory and motor areas of the brain (accounting for hallucinated dream vision and movement) and to the amygdala (accounting for the emotion of dreams).
The specification of these neurochemical differences enables a three-dimensional state space model to be constructed that integrates activation level (A), input-output gating (I) with the brain modulatory factor (M). This hybrid psychophysiological construct thus updates both activation synthesis and reciprocal interaction by representing the energy level (A) information source (I) and processing mode (M) of the brain mind as a single point that continuously moves through the state space as a function of the values of A, I, and M.
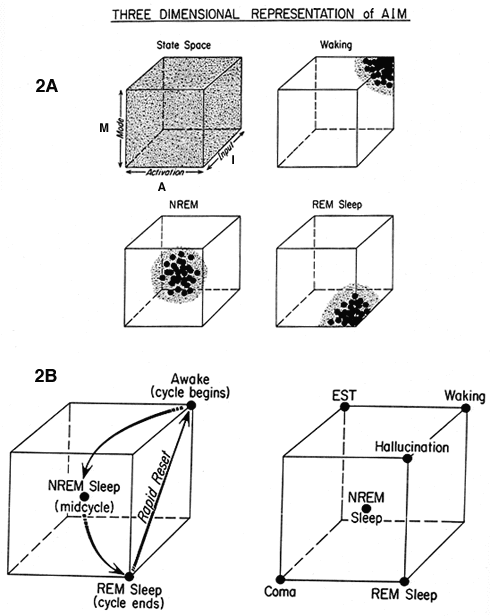
Figure 2a Three-dimensional state space defined by the values for brain activation (A), input source and strength (I), and mode of processing (M). It is theoretically possible for the system to be at any point in the state space, and an infinite number of state conditions is conceivable. In practice the system is normally constrained to a boomerang-like path from the back upper right in waking (high A, I, and M), through the center in NREM (intermediate A, I, and M) to the front lower right in REM sleep (high A, low I, and M).
Figure 2b (A) Movement through the state space during the sleep cycle. (B) Segments of the state space associated with some normal, pathological, and artificial conditions of the brain.
According to AIM, dreaming is most likely to occur when activation is high, when the information source shifts from external to internal, and when the neuromodulatory balance shifts from aminergic to cholinergic. Because these shifts may occur gradually or suddenly, it is not surprising that the correlation of physiology with psychology is also statistical. REM is the most highly conducive to dreaming, but it can also occur at sleep onset and NREM sleep, both of which fulfill some of the necessary physiological conditions.
Our natural skepticism about the relevance of animal model data for human psychophysiology has been partially dispelled by recent POSITRON EMISSION TOMOGRAPHY (PET) studies of the human brain, which reveal significant regional changes in activation level during REM sleep compared to waking. The subjects of these studies all reported dreams after awakening from REM-sleep in the scanner. First and foremost is activation of the pontine brain stem, the presumed organizer of the REM-sleep brain. Second is the selective activation of the limbic forebrain and paralimbic cortex, the supposed mediator of dream emotion. Third is the selective inactivation of the dorsal prefrontal cortex, a brain region essential to self-reflective awareness and to executively guided thought, judgment, and action. Both of these cognitive functions are markedly deficient in dreaming.
Unfortunately, imaging techniques do not have the spatial or molecular resolution necessary to confirm the neuromodulatory hypothesis of AIM. But an extensive body of human psychopharmacological data is consonant with the basic assumptions of the model. Drugs that act as aminergic agonists (or reuptake blockers) first suppress REM and REM-sleep dreaming. When they are later withdrawn, a marked and unpleasant intensification of dreaming and even psychosis may occur. If those drugs also possess anticholinergic actions, the effects on dreaming are even more pronounced. Finally, and most significantly, human REM-sleep dreaming is potentiated by some of the same cholinergic agonist drugs that experimentally enhance REM sleep in animals.
Aserinsky, E., and N. Kleitman. (1953). Regularly occurring periods of eye motility and concomitant phenomena during sleep. Science 118:273-274.
Foulkes, D. (1985). Dreaming: A Cognitive-Psychological Analysis. Mahwah, NJ: Erlbaum.
Freud, S. (1900). The Interpretation of Dreams. Trans. J. Strachey. New York: Basic Books.
Hobson, J. A. (1988). The Dreaming Brain. New York: Basic Books.
Hobson, J. A. (1990). Activation, input source, and modulation: A neurocognitive model of the state of the brain-mind. In R. Bootzin, J. Kihlstrom, and D. Schacter, Eds., Sleep and Cognition. Washington, DC: American Psychological Association, pp. 25-40.
Hobson, J. A. (1994). The Chemistry of Conscious States. Boston: Little Brown.
Hobson, J. A., and R. W. McCarley. (1977). The brain as a dream-state generator: An activation-synthesis hypothesis of the dream process. Am. J. Psychiat. 134:1335-1348.
Hobson, J. A., E. Hoffman, R. Helfand, and D. Kostner. (1987). Dream bizarreness and the activation-synthesis hypothesis. Hu-man Neurobiology 6:157-164.
Llinas, R., and D. Pare. (1991). Commentary on dreaming and wakefulness. Neuroscience 44:521-535.
Solms, M. (1997). The Neuropsychology of Dreams: A Clinico- Anatomical Study. Mahwah, NJ: Erlbaum.