Most of what we know about the neural mechanisms of object recognition in humans has come from the study of agnosia, or impaired object recognition following brain damage. In addition, in recent years, functional neuroimaging in normal humans has begun to offer insights into object recognition. This article reviews both literatures, with greater emphasis accorded to agnosia because of its currently greater contribution to our understanding of human object recognition.
To be considered agnosia, an object recognition impairment must be selective in the sense of not being attributable to impaired elementary perceptual function or general intellectual decline. It must also be a true impairment of recognition, as opposed to an impairment of naming. Agnosias are generally confined to a single perceptual modality, such as auditory (Vignolo 1969), tactile (Reed, Caselli, and Farah 1996), or visual (Farah 1990), suggesting that for each perceptual modality there is a stage of processing beyond elementary perceptual processes that is nevertheless modality-specific, and that represents learned information about objects' sounds, tactile qualities, and visual appearances. In the case of visual agnosia, which is the focus of this article, this stage presumably corresponds to the inferior temporal regions that have been studied using physiological techniques in the monkey (see also OBJECT RECOGNITION, ANIMAL STUDIES). The different types of visual agnosia provide insights into the organization of high-level visual object representations in humans, by showing us the "fracture lines" of the system.
Lissauer (1890) introduced a fundamental distinction between two broad classes of agnosia: those in which perception seemed clearly at fault, which he termed apperceptive, and those in which perception seemed at least roughly intact, which he termed associative. Lissauer hypothesized that the latter type of patient suffered from an inability to associate percepts with meaning. Although the theory behind this classification system is now widely questioned, the classification itself -- that is, the separation of patients with obvious perceptual disorders from patients without obvious perceptual disorders -- has proved useful.
The apperceptive agnosias have received less attention than the associative agnosias, perhaps because they are less surprising or counterintuitive. Although such elementary visual functions as acuity and color perception are roughly intact, higher levels of perception such as visual grouping appear to be disrupted, and the object recognition impairment is secondary to these perceptual impairments. In this article I focus on the associative agnosias because they are the most directly relevant to object recognition per se. Readers may consult chapters 2 and 3 of Farah (1990) for further information on the apperceptive agnosias.
In contrast to the apperceptive agnosias, perception seems roughly normal in associative agnosia, and yet patients cannot recognize much of what they see. Most often, associative agnosia follows bilateral inferior occipitotemporal lesions, although unilateral lesions of either the left or right hemisphere are sometimes sufficient (see the final section). The classic case of Rubens and Benson (1971) shows all of the cardinal signs of associative agnosia, including preserved recognition of objects through modalities other than vision, failure to indicate visual recognition verbally and nonverbally, and apparently good visual perception.
The patient could not identify common objects presented visually, and did not know what was on his plate until he tasted it. He identified objects immediately on touching them. When shown a stethoscope, he described it as "a long cord with a round thing at the end," and asked if it could be a watch. He identified a can opener as "could be a key. . . ." He was never able to describe or demonstrate the use of an object if he could not name it. . . . He could match identical objects but not group objects by categories (clothing, food). He could draw the outlines of objects which he could not identify. . . . Remarkably, he could make excellent copies of line drawings and still fail to name the subject.
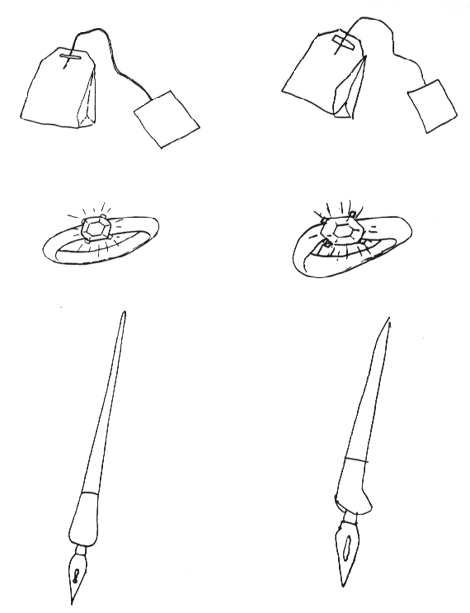
The modality-specific failure of object recognition, in the context of normal intellect, is what one would expect following destruction of the kinds of object representations found at higher levels of the primate visual system, and indeed the most common lesion locations are roughly consistent with this hypothesis (the human lesions are perhaps a bit more posterior). Although the good copies and successful matching performance of associative agnosic patients might seem inconsistent with the hypothesis of a visual perceptual impairment, these perceptual tasks do not require the use of object representations per se. Indeed, the manner in which associative agnosic patients copy and match is consistent with the use of lower-level visual representations in which objects per se are not explicitly represented: they copy line by line, and match feature by feature, unlike normal subjects who organize their copying and matching of these local elements into more global, object-based units (see Farah 1990, chaps. 4 and 5 for a review). The left side of figure 1 shows three drawings that an associative agnosic patient was unable to recognize. When I asked him to copy the drawings, he produced the very adequate copies shown on the right, but only after a laborious, line-by-line process.

Figure 1 Three drawings that an associative agnosic patient could not recognize (left) and the good-quality copies that he was nevertheless able to produce (right).
Studies using POSITRON EMISSION TOMOGRAPHY (PET) and functional MAGNETIC RESONANCE IMAGING (fMRI) have confirmed the most basic conclusion to be drawn from the agnosia literature, namely, that there are visual modality-specific brain regions in inferior temporo-occipital cortex whose function is object perception. Relative to baselines involving the viewing of gratings, random lines, or disconnected object pieces, the viewing of objects is generally associated with temporal or occipital activation, or both, in both hemispheres (e.g., see Menard et al. 1996; Kanwisher et al. 1996, 1997; Sergent, Ohta, and MacDonald 1992; see Aguirre and Farah 1998 for a review). Furthermore, this localization held both for studies that required subjects to perform active information retrieval (e.g., is the depicted object living or nonliving?) and for others that required only passive viewing, suggesting that the critical determinant of the region's activation is object perception, rather than the association of stored memory knowledge with a percept. Indeed, Kanwisher et al. (1997) found no greater activation for unfamiliar objects than for familiar objects, which have a preexisting memory representation.
Agnosia does not always affect all types of stimuli equally. The scope of the deficit varies from case to case, with recognition of faces, objects, and printed words all pairwise dissociable. These dissociations provide us with insights into the internal organization of high-level visual object representation. Similarly, neuroimaging studies have sometimes found differing patterns of activation for different stimulus types (Aguirre and Farah 1998).
When agnosia is confined to faces or is disproportionately severe for faces, it is prosopagnosia. There are many cases of profound FACE RECOGNITION impairment, with little or no evident object agnosia, in the literature. Pallis (1955) provides a detailed case study of a patient whose impairment of face recognition was so severe, he mistook his own reflection in a mirror for a rude stranger staring at him.
Are faces really disproportionately impaired in prosopagnosia, consistent with a distinct subsystem for face recognition, or does the appearance of a selective deficit result from the need for exceedingly fine discrimination among visually similar members of a single category? Recent evidence suggests that there is specialization within the visual system for faces. McNeill and Warrington (1993) showed that a prosopagnosic patient was better able to recognize individual sheep faces than individual human faces, even though normal subjects find the human faces easier to recognize. Farah, Klein, and Levinson (1995) showed that a prosopagnosic patient was disproportionately impaired at face recognition relative to common object recognition, taking into account the difficulty of the stimulus sets for normal subjects. This was true even when the common objects were all eyeglass frames, a large and visually homogeneous category. Farah et al. (1995) showed that the same subject was impaired at upright face perception relative to inverted face perception, even though normal subjects find the latter harder. The existence of patients who are more impaired with objects than with faces also supports the independence of prosopagnosia and object agnosia. Feinberg et al. (1994) documented impaired object recognition in a series of patients with preserved face recognition.
Attempts to dissociate face and object perception using neuroimaging have produced variable results, although in at least some studies the patterns of activation were different (Sergent et al. 1992; Kanwisher et al. 1996).
Orthography-specific processing systems? So-called pure alexics typically read words letter by letter, in a slow and generally error-prone manner. Their impairment is called "pure" because they are able to comprehend spoken words, they have no problem writing words, and their recognition of objects and faces seems normal. Although pure alexia is generally discussed in the context of language and reading disorders, it is clearly also an impairment of visual recognition affecting printed words. Furthermore, in all the cases so far examined, the visual recognition impairment is not confined to words, but also affects the processing of nonorthographic stimuli whenever rapid processing of multiple shapes is required, be they letters in words or sets of abstract shapes (Farah and Wallace 1991; Kinsbourne and Warrington 1962; Levine and Calvanio 1978; Sekuler and Behrmann 1996; see Farah and Wallace 1991 for a discussion of some apparently conflicting data). Although clinical descriptions suggest that in some cases orthographic stimuli may be disproportionately affected, for example, relative to numerical stimuli, this can be understood in terms of segregation of representations for orthographic stimuli within a visual area dedicated to rapid encoding of multiple shapes in general (Farah in press). Polk and Farah (1995) describe and test a mechanism by which such segregation could occur in a self-organizing network, based on the statistics of co-occurrence among letter and nonletter stimuli in the environment.
Just as pure alexia is an impairment of printed word recognition in the absence of obvious impairments of single-object recognition, there are cases of object recognition impairment with preserved reading. For example, the case of Gomori and Hawryluk (1984) was impaired at recognizing a variety of objects and the faces of his friends and family. He nevertheless continued to read with ease, even when interfering lines were drawn across the words. Thus, like prosopagnosia and object agnosia, pure alexia and object agnosia are doubly dissociable.
Only one neuroimaging study has directly compared the patterns of activation evoked by printed words and objects, and did find a degree of separation (Menard et al. 1996).
Localization of high-level object representation in the human visual system: Ironically, although the primary goal of neuroimaging is localization, and agnosia research is subject to the vagaries of naturally occurring lesions, the clearest evidence concerning the anatomy of object recognition comes from patient research. In general, the intrahemispheric location of damage is generally occipitotemporal, involving both gray and white matter. In order to understand the laterality of visual recognition processes, it is crucial to distinguish between subtypes of agnosia. Cases of associative agnosia have been reported following unilateral right hemisphere lesions, unilateral left hemisphere lesions, and bilateral lesions. The dual systems hypothesis presented above helps reduce the variability in lesion site. Agnosic patients presumed to have an impairment of just the first ability (in mild form affecting just faces, in more severe form affecting faces and objects but not words) usually have bilateral inferior lesions, although occasionally unilateral right hemisphere lesions are reported (Farah 1991). Agnosic patients presumed to have an impairment of just the second ability (in mild form affecting just words, in more severe form affecting words and objects but not faces) generally have unilateral left inferior lesions (Farah 1991; Feinberg et al. 1994). Agnosic patients presumed to have an impairment in both abilities (affecting faces, objects, and words) generally have bilateral lesions.
Aside from confirming the generalization that face, object, and VISUAL WORD RECOGNITION tasks involve posterior cortices, the neuroimaging literature tells us little about the localization of different subtypes of object recognition (Aguirre and Farah 1998). The precise locations of areas responsive to faces, nonface objects, and words differ widely, from study to study, within posterior association cortex. Whether this reflects individual variability in brain organization, problems with normalization and statistical procedures for analyzing images of brain activity, or the difference between localizing areas that are activated (as revealed by neuroimaging) vs. areas that are necessary (as revealed by lesions) for visual recognition remains to be discovered.
Aguirre, G. K., and M. J. Farah. (1998). Imaging visual recognition. Trends in Cognitive Sciences to appear.
Farah, M. J. (1990). Visual Agnosia: Disorders of Object Recognition and What They Tell Us About Normal Vision. Cambridge, MA: MIT Press/Bradford Books.
Farah, M. J. (1991). Patterns of co-occurrence among the associative agnosias: Implications for visual object representation. Cognitive Neuropsychology 8:1-19.
Farah, M. J. (Forthcoming). Are there orthography-specific brain regions? Neuropsychological and computational investigations. In R. M. Klein and P. A. McMullen, Eds., Converging Methods for Understanding Reading and Dyslexia. Cambridge, MA: MIT Press.
Farah, M. J., K. L. Klein, and K. L. Levinson. (1995). Face perception and within-category discrimination in prosopagnosia. Neuropsychologia 33:661-674.
Farah, M. J., and M. A. Wallace. (1991). Pure alexia as a visual impairment: A reconsideration. Cognitive Neuropsychology 8:313-334.
Farah, M. J., K. D. Wilson, H. M. Drain, and J. R. Tanaka. (1995). The inverted inversion effect in prosopagnosia: Evidence for mandatory, face-specific perceptual mechanisms. Vision Research 35:2089-2093.
Feinberg, T. E., R. J. Schindler, E. Ochoa, P. C. Kwan, and M. J. Farah. (1994). Associative visual agnosia and alexia without prosopagnosia. Cortex 30:395-411.
Gomori, A. J., and G. A. Hawryluk. (1984). Visual agnosia without alexia. Neurology 34:947-950.
Kanwisher, N., M. M. Chun, J. McDermott, and P. J. Ledden. (1996). Functional imaging of human visual recognition. Cognitive Brain Research 5:55-67.
Kanwisher, N., R. Woods, M. Ioacoboni, and J. Mazziotta. (1997). A locus in human extrastriate cortex for visual shape analysis. Journal of Cognitive Neuroscience 9:133-142.
Kinsbourne, M., and E. K. Warrington. (1962). A disorder of simultaneous form perception. Brain 85:461-486.
Levine, D. N., and R. Calvanio. (1978). A study of the visual defect in verbal alexia-simultanagnosia. Brain 101:65-81.
Lissauer, H. (1890). Ein Fall von Seelenblindheit nebst einem Beitrag zur Theorie derselben. Archiv für Psychiatrie und Nervenkrankheiten 21:222-270.
McNeil, J. E., and E. K. Warrington. (1993). Prosopagnosia: A face-specific disorder. Quarterly Journal of Experimental Psychology A. Human Experimental Psychology 46:1-10.
Menard M. T., S. M. Kosslyn, W. L. Thompson, N. M. Alpert, and S. L. Rauch. (1996). Encoding words and pictures: A positron emission tomography study. Neuropsychologia 34:185-194.
Pallis, C. A. (1955). Impaired identification of faces and places with agnosia for colors. Journal of Neurology, Neurosurgery and Psychiatry 18:218-224.
Polk, T. A., and M. J. Farah. (1995). Brain localization for arbitrary stimulus categories: A simple account based on Hebbian learning. Proceedings of the National Academy of Sciences 92:12370-12373.
Reed, C. L., R. Caselli, and M. J. Farah. (1996). Tactile agnosia: Underlying impairment and implications for normal tactile object recognition. Brain 119:875-888.
Rubens, A. B., and D. F. Benson. (1971). Associative visual agnosia. Archives of Neurology 24:305-316.
Sekuler, E., and M. Behrmann. (1996). Perceptual cues in pure alexia. Cognitive Neuropsychology 13:941-974.
Sergent, I., S. Ohta, and B. MacDonald. (1992). Functional neuroanatomy of face and object processing. Brain 115:15-36.
Vignolo, L. A. (1969). Auditory agnosia. In A. L. Benton, Ed., Contributions to Clinical Neuropsychology. Chicago: Aldine.