Much as other systems with a historic origin (e.g., the reticular system), the limbic system (LS) is difficult to define as it has gone through numerous modifications, adaptations, refinements, and expansions during the more than 100 years of its existence. Furthermore, problems with its description arise from the facts that it is frequently composed of only portions of larger units (e.g., only a minority of the thalamic nuclei are included), and that it varies considerably among species (e.g., the olfactory system, a portion of the LS, first expands considerably in mammals as opposed to nonmammals such as birds, but then shrinks again in whales and primates). Basically the LS constitutes an agglomerate of brain structures with a cortical core around the corpus callosum and within the medial temporal lobe and with a number of subcortical structures extending from the hindbrain to the forebrain (figure 1). Many of these structures are central to the processing of EMOTIONS and MEMORY, including the evaluation of sensory functions such as PAIN.

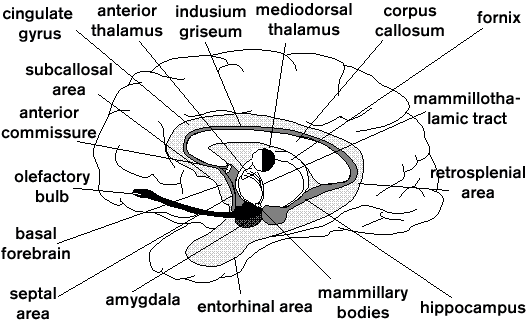
Figure 1. Schematic section through the forebrain (i.e., without the brain stem) showing the ringlike arrangement of the limbic structures around the corpus callosum and below it. The Papez circuit is formed principally by the hippocampus, mammillary bodies, anterior thalamus, cingulate gyrus, and is interconnected via the fornix, mammillothalamic tract, fibers from the anterior thalamus to the cingulate gyrus and to portions of the hippocampal region, and the cingulum fibers that run near the indusium griseum, an extension of the hippocampal formation (Irle and Markowitsch 1982). All other structures mentioned are usually regarded as belonging to the limbic system as well. Some brain stem nuclei might be added.
The term le grand lobe limbique was coined by BROCA (1878) as an anatomical structure. Broca and his contemporaries nevertheless thought that the limbic structures were largely olfactory and might therefore be subsumed under the term rhinencephalon (cf. Laurent 1997 for olfactory processing). Later research shifted the dominant functional implications of the LS to the processing of emotions and memory and modified the regions to be subsumed under this term. This discussion has continued until today (Papez 1937; MacLean 1952, 1970; Nauta 1979; LeDoux 1996; Nieuwenhuys 1996). While the LS (the term was introduced by MacLean 1952) is frequently regarded as an ancient brain system which regresses during phylogeny, numerous more recent studies have shown that, on the contrary -- with the exception of the olfactory regions -- most structures of this system expand and increase in differentiation (e.g., Stephan 1975; Armstrong 1986). (This expansion is, however, less prominent than that of neocortical areas.)
Based on comparative anatomy and evolutionary theory, MacLean (1970) divided the brain into three general compartments: (1) a protoreptilian portion (spinal cord, parts of the midbrain and diencephalon, BASAL GANGLIA); (2) a paleomammalian one -- in principle the LS -- , and (3), a neomammalian one, largely the neocortical mantle. The LS therefore constitutes a link between the oldest and the newest attributes of the mammalian, in particular the human, brain. It includes (1) the limbic cortex, a circumscribed cortical region along the medial rim of the cerebrum; (2) the limbic nuclei of the tel-, di-, and mesencephalon; and (3) the fiber tracts interconnecting these structures. The limbic cortex is further subdividable into an inner ("allocortical," i.e., constituted of the phylogenetically oldest, three-layered cortex) and an outer ring ("juxtallocortical," i.e., constituted of transitional, four- or five-layered cortex; Isaacson 1982).
The core of the LS is included in the so-called Papez circuit (Papez 1937) or medial limbic circuit (see figure 1). This circuit is primarily engaged in the transfer of information from short-term to long-term memory. Another circuit that is more closely related to emotional processing but still relevant to mnemonic information processing as well is the basolateral limbic circuit, or lateral limbic circuit (Sarter and Markowitsch 1985). It is constituted by the triangle formed by the amygdala, the mediodorsal thalamic nucleus, and the basal forebrain regions. Interconnecting fibers are the ventral amygdalofugal pathway, the anterior thalamic peduncle, and the bandeletta diagonalis (the circuit is depicted in figure 48.4 of Markowitsch 1995).
As mentioned above, there have been various attempts to expand the LS (Isaacson 1982; Nauta 1979; Nieuwenhuys 1996). All of these nevertheless agree in principle with MacLean's (1970) proposal to see this system as the mediator between the neocortical mantle (dealing with sensory processing, memory storage, and the initiation and supervision of behavior) and the "lower," largely motoric regions of the brain stem and the basal ganglia.
Though the structures of the LS are predominantly involved in emotional, motivational, and memory-related aspects of behavior, some subclustering should be noted: the septal region, the amygdala, and the cingulate cortex are largely engaged in the control of emotions ranging from heightened ATTENTION and arousal to rage and aggression. In evaluating emotions the septum may partly act in opposition to amygdala and cingulate cortex. The amygdala is furthermore involved in motivational regulations and in evaluating information of biological or social significance (and therefore indirectly in memory processing). Damage to the amygdala may result in conditions of tameness, hypersexuality, amnesia, agnosia, aphagia, and hyperorality (Klüver-Bucy syndrome). The hippocampal formation and surrounding structures are principally engaged in transferring memories from short-term to long-term storage, but do have additional functions (e.g., in the spatial and possibly also in the time domains). Anterior and medial nuclei of the thalamus and the mammillary body of the hypothalamus control memory transfer as well ("bottleneck structures"; Markowitsch 1995). Also, these nuclear configurations (to which nonspecific thalamic nuclei belong as well) control further forms of behavior ranging from sleep to possibly consciousness. Between different species, functional shifts of limbic structures have been noted.
There is consequently both functional unity and diversity within the LS. As an example, it is still largely unknown whether the medial temporal lobe structures (with the hippocampus as core) and the medial diencephalic structures (medial and anterior thalamus, mammillary bodies) constitute one or two memory systems. One reason for this uncertainty can be sought in the multitude of fiber bundles interconnecting LS structures in an extensive network. High-resolution dynamic imaging research (e.g., POSITRON EMISSION TOMOGRAPHY) may provide answers in the near future.
Armstrong, E. (1986). Enlarged limbic structures in the human brain: the anterior thalamus and medial mamillary body. Brain Research 362:394-397.
Broca, P. (1878). Anatomie comparée des circonvolutions cérébrales. Le grand lobe limbique et la scissure limbique dans le série des mammifières. Revue Anthropologique 2:385-498.
Irle, E., and H. J. Markowitsch. (1982). Connections of the hippocampal formation, mamillary bodies, anterior thalamus and cingulate cortex. A retrograde study using horseradish peroxidase in the cat. Experimental Brain Research 47:79-94.
Isaacson, R. L. (1982). The Limbic System. 2nd ed. New York: Plenum Press.
Laurent, G. (1997). Olfactory processing: maps, time and codes. Current Opinion in Neurobiology 7:547-553.
LeDoux, J. E. (1996). The Emotional Brain. New York: Simon and Schuster.
MacLean, P. D. (1952). Some psychiatric implications of physiological studies of frontotemporal portion of limbic system (visceral brain). Electroencephalography and Clinical Neurophysiology 4:407-418.
MacLean, P. D. (1970). The triune brain, emotion and scientific bias. In F. O. Schmitt, Ed., The Neurosciences: Second Study Program. New York: Rockefeller University Press, pp. 336-348.
Markowitsch, H. J. (1995). Anatomical basis of memory disorders. In M. S. Gazzaniga, Ed., The Cognitive Neurosciences. Cambridge, MA: MIT Press, pp. 665-679.
Nauta, W. J. H. (1979). Expanding borders of the limbic system concept. In T. Rasmussen and R. Marino, Eds., Functional Neurosurgery. New York: Raven Press, pp. 7-23.
Nieuwenhuys, R. (1996). The greater limbic system, the emotional motor system and the brain. Progress in Brain Research 107:551-580.
Papez, J. W. (1937). A proposed mechanism of emotion. Archives of Neurology and Psychiatry 38:725-743.
Sarter, M., and H. J. Markowitsch. (1985). The amygdala's role in human mnemonic processing. Cortex 21:7-24.
Stephan, H. (1975). Allocortex. Handbuch der mikroskopischen Anatomie des Menschen, vol. 4, part 9. Berlin: Springer-Verlag.
Cahill, L., R. Babinsky, H. J. Markowitsch, and J. L. McGaugh. (1995). Involvement of the amygdaloid complex in emotional memory. Nature 377:295-296.
Cramon, D.Y. von, H. J. Markowitsch, and U. Schuri. (1993). The possible contribution of the septal region to memory. Neuropsychologia 31:1159-1180.
Groenewegen, H. J., C. I. Wright, and A. V. J. Beijer. (1996). The nucleus accumbens: gateway for limbic structures to reach the motor system? Progress in Brain Research 107:485-511.
Lilly, R., J. L. Cummings, D. F. Benson, and M. Frankel. (1983). The human Klüver-Bucy syndrome. Neurology 33:1141-1145.
Macchi, G. (1989). Anatomical substrate of emotional reactions. In L. R. Squire and G. Gainotti, Eds., Handbook of Neuropsychology, vol. 3. Amsterdam: Elsevier, pp. 283-304.
Markowitsch, H. J., P. Calabrese, M. Würker, H. F. Durwen, J. Kessler, R. Babinsky, D. Brechtelsbauer, L. Heuser, and W. Gehlen. (1994). The amygdala's contribution to memory -- a PET-study on two patients with Urbach-Wiethe disease. Neuroreport 5:1349-1352.
Mesulam, M.-M. (1985). Patterns in behavioral neuroanatomy: association areas, the limbic system, and behavioral specialization. In M.-M. Mesulam, Ed., Principles of Behavioral Neurology. Philadelphia: F. A. Davis, pp. 1-70.
Reep, R. (1984). Relationship between prefrontal and limbic cortex: a comparative anatomical review. Brain, Behavior and Evolution 25:5-80.
Schneider, F., R. E. Gur, L. H. Mozley, R. J. Smith, P. D. Mozley, D. M. Censits, A. Alavi, and R. C. Gur. (1995). Mood effects on limbic blood flow correlate with emotional self-rating: a PET study with oxygen-15 labeled water. Psychiatry Research: Neuroimaging 61:265-283.
Scoville, W. B., and B. Milner. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry 20:11-21.
Tulving, E., and H. J. Markowitsch. (1997). Memory beyond the hippocampus. Current Opinion in Neurobiology 7:209-216 .