Figure 1
Like all parts of the CEREBRAL CORTEX, the function of visual cortex is dependent on the organization of its connections, the types of synapses they form, and how postsynaptic neurons respond to and integrate synaptic inputs. The various neurons within the cerebral cortex can be classified based on differences in any of these traits and their unique relationships to cortical circuits.
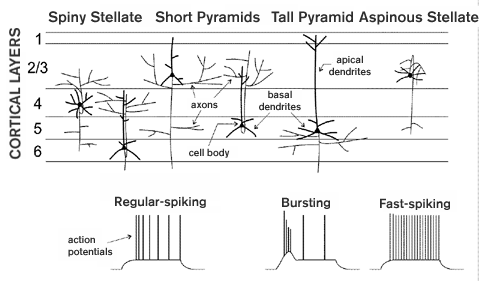
Numerous neuronal types are found in visual cortex as well as other cortical areas. The actual categorization of individual cells is, of course, highly dependent on the definitions used to distinguish them. Among many possibilities, cells can be defined in terms of the functional influence of their synapses (excitatory or inhibitory, strong or weak); anatomical features (spiny or aspinous dendrites, spiny stellate or pyramidal dendritic morphology; see figure 1); laminar position (the cortical layer containing the cell body; see figure 1); intrinsic membrane properties (fast-spiking, regular- spiking, bursting; see figure 1); or patterns of connectivity. In the most clear-cut cases, many of these definitions generate the same groupings. Most notably, inhibitory and excitatory neurons compose two distinct groups on the basis of several features. They release different NEUROTRANSMITTERS and their synapses therefore have different functional influences (g-aminobutyric acid, inhibitory vs. glutamate, excitatory). Inhibitory neurons also have aspinous dendrites and are fast-spiking compared to excitatory neurons which have spiny dendrites and are regular-spiking or bursting (see figure 1 and below).
Although inhibitory cells account for only about fifteen to twenty percent of visual cortical neurons, they are a highly diverse population. They have been distinguished experimentally based primarily on morphology. By such criteria more than a dozen different types of aspinous neurons can be identified. In a few cases, important functional implications can be inferred from the anatomy. For example, "chandelier" cells form inhibitory synapses onto the axon initial segments (where the axon leaves the cell body; see figure 1) of spiny neurons and can therefore veto their output. But most inhibitory connections probably have more subtle influences via connections onto dendrites, where they interact with nearby excitatory connections. A better understanding of the functional importance of most of the morphological distinctions awaits further study.
The great majority of visual cortical neurons (about 80-85 percent) are spiny and therefore excitatory. Outside of primary visual cortex (V1), virtually all of these have a pyramidal dendritic morphology (see figure 1). Such cells have a long apical dendrite extending from the cell body "up" toward more superficial layers (i.e., layer 1), as well as more numerous, shorter basal dendrites extending downward and obliquely. Thus, the top of the apical dendrite and bottoms of the most lateral basal dendrites define the corners of the "pyramid." Pyramidal neurons can be further distinguished by variations in their patterns of dendritic branching. Such differences are often correlated with different intrinsic physiological properties or patterns of connectivity. For example "tall" pyramids have long apical dendrites and often fire action potentials in bursts (see figure 1 and below). A second type of spiny neuron, the spiny stellate, is found in layer 4 of V1. These lack a prominent apical dendrite and instead have numerous shorter dendrites, extending obliquely upward as well as downward, to define a roughly spherical volume (see figure 1).
Cortical neurons also vary and can be classified according to intrinsic physiological differences. These differences are reflected in the patterns and shapes of action potentials that are generated on the injection of electrical current into the cell body (see figure 1). Inhibitory neurons fire high- frequency trains of brief action potentials. They are thus termed fast-spiking. Spiny neurons have broader, less brief action potentials and in most cases the firing rate decreases gradually with each spike. These are regular-spiking neurons. Bursting pyramidal neurons are rarer and fire action potentials in groups rather than singly. Bursting neurons are typically found in deep cortical layers and are also distinct from regular-spiking neurons in terms of both their dendritic morphology and axonal connections (see below).

A conspicuous feature of all cortical areas is their laminar organization. Layers are apparent in cross sections through the cortical sheet as regions with varying densities and sizes of neurons. The layers are numbered 1 to 6, with layer 1 located most superficially, at the outer surface, and layer 6 the deepest (see figure 1). Grouping of cortical neurons according to laminar position is straightforward and also useful since the most prominent feature of connectivity in visual cortex is its laminar organization. The laminar specificity of connections is a consequence of the laminar stratification of axonal arbors (see figure 1 and below). Thus, the laminar position of a neuron's cell body tends to be highly correlated with its connectivity. This is particularly true of aspinous, inhibitory neurons and spiny stellate neurons since their dendrites, which receive connections from the axons of other neurons, are usually confined to a single layer. But dendritic arbors of pyramidal neurons can span several layers, with apical dendritic branches also being highly stratified. Laminar specificity is a characteristic of both the corticocortical connections between the numerous visual cortical areas and local connections within a cortical area. And connections with subcortical structures also arise from and terminate in distinct cortical layers.
The laminar patterns of connections between visual cortical areas are closely correlated with hierarchical relationships between areas. At the bottom of the hierarchy is the primary visual area, V1. Virtually all of the visual information reaching the cortex is first channeled through this area, where it is processed before being sent on to higher areas. Corticocortical connections are made by pyramidal neurons and are therefore excitatory. Forward connections, from lower to higher areas in the hierarchy, originate in superficial layers (layers 2 and 3) and terminate in the middle layer (layer 4). (In the case of V1 the forward input to layer 4 originates from the THALAMUS.) Feedback connections originate from deep-layer (layer 5 or 6 or both) neurons, and the axons of these cells terminate in superficial and deep layers. The forward connections are strong and their organization has a dominant influence on the visual responses of recipient neurons (see SINGLE-NEURON RECORDING). Feedback connections, although generally more numerous, are functionally weaker and serve to modulate responses driven by the forward connections.
Local excitatory connections, intrinsic to a single visual cortical area, are also highly layer-specific and can be classified as forward, dominant and feedback, modulatory. This organization is most clear in V1, where the connections are understood in the greatest detail. As noted above, the dominant, forward input to a cortical area targets layer 4. Locally, these layer 4 neurons provide dominant, forward input via their axonal arbors to layers 2 and 3 (e.g., spiny stellates in layer 4 of V1, see figure 1). And these layer 2 and 3 neurons in turn provide the feedforward output to higher cortical areas (see above). Thus, there are two levels of local forward processing (layer 4 and layers 2 and 3). Each of these levels or layers also receives local, modulatory feedback from the axons of deep-layer cells (see figure 1). Layer 6 provides feedback to its partner, layer 4; and layer 5 to its partner, layers 2 and 3. The deep layers providing this feedback receive weaker forward input from the same sources as their partner plus from the partner itself (see figure 1). They therefore incorporate information about the input to and output from their partner and modulate the partner's activity with their feedback connections.
Visual cortical areas also interact extensively with subcortical areas, most notably the thalamus, superior colliculus, and visual claustrum. These connections are also layer- specific. Thalamic nuclei, including the lateral geniculate nucleus and pulvinar nucleus, are composed of anatomically and functionally distinct subdivisions, each of which connects to distinct visual cortical areas and layers (see VISUAL ANATOMY AND PHYSIOLOGY). Connections from visual cortex to subcortical targets originate from neurons in deep layers. Unlike corticocortical connections from deep layers, however, these do not necessarily constitute modulatory, feedback connections. They arise from populations of neurons different from those that make corticocortical or local feedback connections and their input from superficial layers might be stronger. For example, in V1, connections from layer 5 to the superior colliculus or from layer 6 to the visual claustrum arise from neurons with longer apical dendrites ("tall" pyramids) and different intrinsic firing properties than those making corticocortical connections.
Since visual cortex offers many experimental advantages and has been more extensively studied than other cortical areas, it is understood in greater detail. It is expected, however, that many of the cell types and principles of connectivity that are revealed here will be applicable to the cerebral cortex as a whole.
Callaway, E. M. (1998). Local circuits in primary visual cortex of the macaque monkey. Annu. Rev. Neurosci. 21:47-74.
Connors, B. W., and M. J. Gutnick. (1990). Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 13:99-104.
Felleman, D. J., and D. C. Van Essen. (1991). Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex 1:1-47.
Gilbert, C. D. (1983). Microcircuitry of the visual cortex. Ann. Rev. Neurosci. 6:217-247.
Kasper, E. M., A. U. Larkman, J. Lubke, and C. Blakemore. (1994). Pyramidal neurons in layer 5 of the rat visual cortex. 1. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J. Comp. Neurol. 339:459-474.
Katz, L. C. (1987). Local circuitry of identified projection neurons in cat visual cortex brain slices. J. Neurosci. 7:1223-1249.
Lund, J. S. (1988). Anatomical organization of macaque monkey striate visual cortex. Ann. Rev. Neurosci. 11:253-288.
Martin, K. A. C. (1984). Neuronal circuits in cat striate cortex. Cereb. Cortex 2:241-284.
Salin, P. A., and J. Bullier. (1995). Corticocortical connections in the visual system: Structure and function. Physiol. Rev. 75:107-154.
Valverde, F. (1985). The organizing principles of the primary visual cortex in the monkey. Cereb. Cortex 3:207-257.
Anderson, J. C., R. J. Douglas, K. A. C. Martin, and J. C. Nelson. (1994). Map of the synapses formed with the dendrites of spiny stellate neurons of cat visual cortex. J. Comp. Neurol. 341:25-38.
Bullier, J., J. M. Hupe, A. James, and P. Girard. (1996). Functional interactions between areas V1 and V2 in the monkey. J. Physiol.(Paris) 90:217-220.
Callaway, E. M., and A. K. Wiser. (1996). Contributions of individual layer 2-5 spiny neurons to local circuits in macaque primary visual cortex. Vis. Neurosci. 13: 907 - 922.
Lund, J. S. (1987). Local circuit neurons of macaque monkey striate cortex: 1. Neurons of laminae 4C and 5A. J. Comp. Neurol. 257:60-92.
Lund, J. S., M. J. Hawken, and A. J. Parker. (1988). Local circuit neurons of macaque monkey striate cortex: 2. Neurons of laminae 5B and 6. J. Comp. Neurol. 276:1-29.
Lund, J. S., and T. Yoshioka. (1991). Local circuit neurons of macaque monkey striate cortex: Neurons of laminae 4B, 4A, and 3B. J. Comp. Neurol. 311:234-258.
Martin, K. A. C., and D. Whitteridge. (1984). Form, function and intracortical projections of spiny neurons in the striate cortex of the cat. J. Physiol. 353:463-504.
Rockland, K. S., and D. N. Pandya. (1979). Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179:3-20.
Stratford, K. J., K. Tarczy-Hornoch, K. A. C. Martin, N. J. Bannister, and J. J. Jack. (1996). Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382:258-261.
Wiser, A. K., and E. M. Callaway. (1996). Contributions of individual layer 6 pyramidal neurons to local circuitry in macaque primary visual cortex. J. Neurosci. 16:2724-2739.