Figure 1
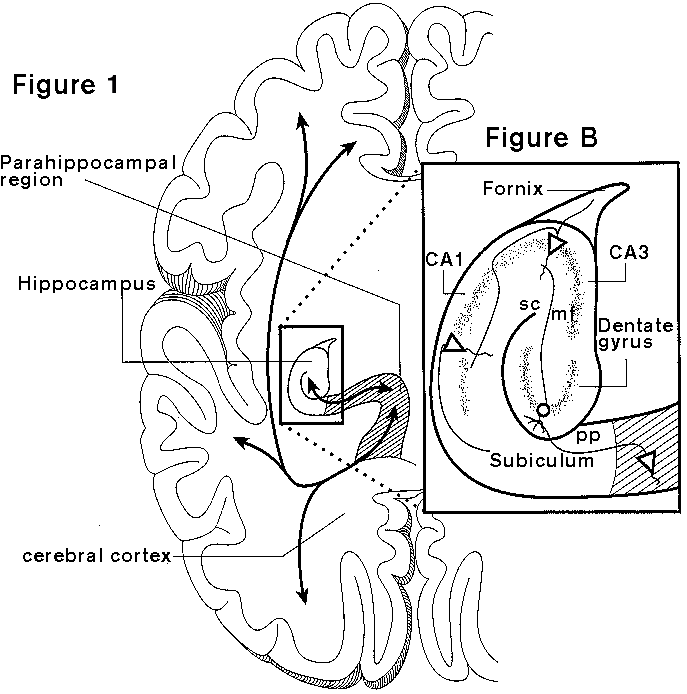
The hippocampus is a brain structure located deep within the temporal lobe, surrounded by the lateral ventricle, and connected to subcortical nuclei via the fornix and to the neocortex via the parahippocampal region. Considerations of the information-processing functions of the hippocampus highlight its position as the final convergence site for outputs from many areas of the CEREBRAL CORTEX, and its divergent outputs that return to influence or organize cortical memory representations (figure 1).

Figure 1
The neocortex provides information to the hippocampus only from the highest sensory processing areas, plus multimodal and LIMBIC SYSTEM cortical areas and the olfactory cortex. These inputs follow a coarse rostral-to-caudal topography arriving in the parahippocampal region, composed of the perirhinal, parahippocampal, and entorhinal cortices (Burwell, Witter, and Amaral 1995). The latter areas project onto the hippocampus itself at each of its main subdi-visions, the dentate gyrus, the CA3 and CA1 components of Ammon's horn, and the subiculum (figure 1). The main flow of information through the hippocampus involves serial connections from the dentate gyrus to CA3, CA3 to CA1, and then CA1 to the subiculum (Amaral and Witter 1989). The intrinsic hippocampal pathway partially preserves the topographical gradients of neocortical input, but there is also considerable divergence and associational connections particularly at the CA3 stage. Outputs of the subiculum, and to a lesser extent CA1, are directed back to the parahippocampal region, which in turn projects back onto the neocortical and olfactory areas that were the source of cortical inputs. These aspects of hippocampal organization maximize the potential for association of information from many cortical streams, and the potential for such associations to influence cortical processing broadly. Furthermore, the capacity for associative plasticity in the form of LONG-TERM POTENTIATION at dentate and CA1 synapses is well established, and has been related to normal rhythmic (theta) bursting activity in the hippocampus and to hippocampal memory function.if
In 1957 Scoville and Milner described a patient known as H. M. who suffered profound amnesia following bilateral removal of substantial portions of both the hippocampus and parahippocampal region. H. M. demonstrated an almost complete failure to learn new material, whereas his remote autobiographical memories and short term memory were completely intact, leading to the view that the hippocampal region plays a specific role in the consolidation of short term memories into a permanent store. In addition, the amnesic impairment is also selective to declarative or explicit memory (cf. IMPLICIT VS. EXPLICIT MEMORY), the capacity for conscious and direct expression of both episodic and semantic memory (Corkin 1984; Squire et al. 1993; see also EPISODIC VS. SEMANTIC MEMORY). Conversely, amnesiacs demonstrate normal MOTOR LEARNING and CONDITIONING, and normal sensory adaptations and "priming" of perceptual stimuli; such forms of implicit memory occur despite their inability to recall or recognize the learning materials or the events surrounding the learning experience (see MEMORY, HUMAN NEUROPSYCHOLOGY). The development of a nonhuman primate model has demonstrated a parallel dissociation between severely impaired recognition memory and preserved acquisition of motor skills and perceptual discriminations following damage to the same hippocampal areas removed in H. M. (see MEMORY, ANIMAL STUDIES).
A central open question is precisely what role the hippocampus plays in declarative memory processing. Studies of the consequences of hippocampal damage in animals have generated several proposals about hippocampal function, each suggesting a specific form of hippocampal-dependent and hippocampal-independent memory. Perhaps the most prominent of these is the hypothesis that the hippocampus constitutes a COGNITIVE MAP, a representation of allocentric space (O'Keefe and Nadel 1978). This notion captures the multimodal nature of hippocampal inputs and accounts for deficits in place learning observed following hippocampal damage. However, this view does not account for the global amnesia observed in humans or for impairments observed on some nonspatial learning tasks in animals with hippocampal damage. A reconciliation of its declarative and spatial functions may be possible by considering a fundamental role for the hippocampus in representing relations among items in a memory network and in "flexibility" of memory expression by which all items can be accessed through any point in the network (Dusek and Eichenbaum 1997). Recent findings using both monkeys and rats have shown that animals with hippocampal damage are severely impaired when challenged to express learned relations between items in a flexible way, and the lack of such flexibility is characteristic of human amnesia (see Eichenbaum 1997; Squire 1992) .
Complementary evidence about the memory processing accomplished by the hippocampus has been derived from studies of the firing patterns of cortical and hippocampal neurons in behaving animals. Recordings at successive cortical stages leading to the hippocampus reflect increasing sensory convergence, from the encoding of specific perceptual features or movement parameters in early cortical areas to that of increasingly complicated and multimodal objects and behaviors at higher cortical stages. Consistent with the view that the hippocampus is the ultimate stage of hierarchical processing, the functional correlates of hippocampal cells are "supramodal" in that they appear to encode the abstract stimulus configurations that are independent of any particular sensory input. Most prominent among the functional types of hippocampal principal neurons are cells that fire selectively when a rat is in a particular location in its environment as defined by the spatial relations among multiple and multimodal stimuli (O'Keefe 1976). The firing of such "place cells" is characteristically not dependent upon any particular stimulus element and is not affected even if all the stimuli are removed, so long as the animal behaves as if it is in the same environment. However, hippocampal neuronal activity is not limited to the encoding of spatial cues, but has also been related to meaningful movement trajectories and actions in rats as well as conditioned motor responses in restrained rabbits and monkeys. In addition, across a variety of learning tasks hippocampal neurons are activated by relevant olfactory, visual, tactile, or auditory cues, and these encodings prominently reflect nonallocentric spatial, temporal, and other relations among the cues that guide performance (Wood, Dudchencko, and Eichenbaum, 1999; Deadwyler and Hampson 1997). These findings extend the range of hippocampal coding to reflect its global involvement in memory and serve to reinforce the conclusion that the hippocampus supports relational representations.
Efforts to understand how hippocampal circuitry mediates memory processing have focused on special aspects of hippocampal architecture: a high convergence of sensory information onto the hippocampus, sparse connectivity within the broad serial divergence and associative connections across the cell population, recurrent connections that characterize dentate gyrus and CA3 pyramidal cells, the small fraction of excited afferent fibers required to drive CA1 cells, and rapid adjustments of synaptic weights at each stage via long term potentiation. These anatomical and physiological features have been simulated in artificial associative networks employed to accomplish distributed recodings of inputs and to perform basic computations that are reflected in hippocampal neural activity (see Gluck 1996). Some models have focused on the central features of cognitive maps, such as the ability to solve problems from partial information, take shortcuts, and navigate via novel routes. Other models have focused on sequence prediction that employs the recall of temporal patterns to accomplish spatial and nonspatial pattern completion and disambiguation, and more generally show how such network memory representations can provide the flexibility of memory expression conferred by the hippocampus.
Amaral, D. G., and M. P. Witter. (1989). The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31:571-591.
Burwell R. D., M. P. Witter, and D. G. Amaral. (1995). Perirhinal and postrhinal cortices in the rat: a review of the neuroanatomical literature and comparison with findings from the monkey brain. Hippocampus 5:390-408.
Deadwyler, S. A., and R. E. Hampson. (1997). The significance of neural ensemble codes during behavior and cognition. Annual Review of Neuroscience 20:217-244.
Dusek, J. A., and H. Eichenbaum. (1997). The hippocampus and memory for orderly stimulus relations. Proceedings of the National Acadamy of Sciences USA 94:7109-7114.
Eichenbaum, H. (1997). Declarative memory: insights from cognitive neurobiology. Annual Review of Psychology 48:547-572.
Gluck, M. A., Ed. (1996). Computational models of hippocampal function in memory. Special Issue of Hippocampus, vol. 6, no. 6.
O'Keefe, J. A. (1976). Place units in the hippocampus of the freely moving rat. Experimental Neurology 51:78-109.
O'Keefe, J., and L. Nadel. (1978). The Hippocampus as a Cognitive Map. New York: Oxford University Press.
Scoville, W. B., and B. Milner. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry 20:11-12.
Squire, L. R. (1992). Memory and the hippocampus: a synthesis of findings with rats, monkeys, and humans. Psychological Reviews 99:195-231.
Squire, L. R., B. Knowlton, and G. Musen. (1993). The structure and organization of memory. Annual Review of Psychology 44:453-495.
Wood, E. R., P. A. Dudchencko, and H. Eichenbaum. (1999). The global record of memory in hippocampal neuronal activity. Nature (in press).
Cohen, N. J., and H. Eichenbaum. (1993). Memory, Amnesia, and the Hippocampal System. Cambridge, MA: MIT Press .